Differentiated evolutionary rates in alternative exons and the implications for splicing regulation
- PMID: 16792801
- PMCID: PMC1543662
- DOI: 10.1186/1471-2148-6-50
Differentiated evolutionary rates in alternative exons and the implications for splicing regulation
Abstract
Background: Alternatively spliced exons play an important role in the diversification of gene function in most metazoans and are highly regulated by conserved motifs in exons and introns. Two contradicting properties have been associated to evolutionary conserved alternative exons: higher sequence conservation and higher rate of non-synonymous substitutions, relative to constitutive exons. In order to clarify this issue, we have performed an analysis of the evolution of alternative and constitutive exons, using a large set of protein coding exons conserved between human and mouse and taking into account the conservation of the transcript exonic structure. Further, we have also defined a measure of the variation of the arrangement of exonic splicing enhancers (ESE-conservation score) to study the evolution of splicing regulatory sequences. We have used this measure to correlate the changes in the arrangement of ESEs with the divergence of exon and intron sequences.
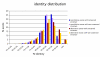
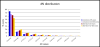
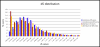
Results: We find evidence for a relation between the lack of conservation of the exonic structure and the weakening of the sequence evolutionary constraints in alternative and constitutive exons. Exons in transcripts with non-conserved exonic structures have higher synonymous (dS) and non-synonymous (dN) substitution rates than exons in conserved structures. Moreover, alternative exons in transcripts with non-conserved exonic structure are the least constrained in sequence evolution, and at high EST-inclusion levels they are found to be very similar to constitutive exons, whereas alternative exons in transcripts with conserved exonic structure have a dS significantly lower than average at all EST-inclusion levels. We also find higher conservation in the arrangement of ESEs in constitutive exons compared to alternative ones. Additionally, the sequence conservation at flanking introns remains constant for constitutive exons at all ESE-conservation values, but increases for alternative exons at high ESE-conservation values.
Conclusion: We conclude that most of the differences in dN observed between alternative and constitutive exons can be explained by the conservation of the transcript exonic structure. Low dS values are more characteristic of alternative exons with conserved exonic structure, but not of those with non-conserved exonic structure. Additionally, constitutive exons are characterized by a higher conservation in the arrangement of ESEs, and alternative exons with an ESE-conservation similar to that of constitutive exons are characterized by a conservation of the flanking intron sequences higher than average, indicating the presence of more intronic regulatory signals.
Figures





References
-
- Cline MS, Shigeta R, Wheeler RL, Siani-Rose MA, Kulp D, Loraine AE. The effects of alternative splicing on transmembrane proteins in the mouse genome. Pac Symp Biocomput. 2004:17–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

