Retinal waves trigger spindle bursts in the neonatal rat visual cortex
- PMID: 16793880
- PMCID: PMC6673818
- DOI: 10.1523/JNEUROSCI.0752-06.2006
Retinal waves trigger spindle bursts in the neonatal rat visual cortex
Abstract
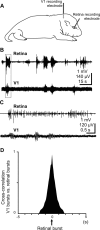
During visual system development, the light-insensitive retina spontaneously generates waves of activity, which are transmitted to the lateral geniculate nucleus. The crucial question is whether retinal waves are further transmitted to the cortex and influence the early cortical patterns of activity. Using simultaneous recordings from the rat retina and visual cortex during the first postnatal week in vivo, we found that spontaneous retinal bursts are correlated with spindle bursts (intermittent network bursts associated with spindle-shape field oscillations) in the contralateral visual cortex (V1). V1 spindle bursts could be evoked by electrical stimulation of the optic nerve. Intraocular injection of forskolin, which augments retinal waves, increased the occurrence of V1 spindle bursts. Blocking propagation of retinal activity, or removal of the retina reduced the frequency, but did not completely eliminate the cortical spindle bursts. These results indicate that spontaneous retinal waves are transmitted to the visual cortex and trigger endogenous spindle bursts. We propose that the interaction between retinal waves and spindle bursts contributes to the development of visual pathways to the cortex.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
