Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses
- PMID: 16793885
- PMCID: PMC6673823
- DOI: 10.1523/JNEUROSCI.0514-06.2006
Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses
Abstract
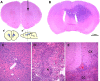
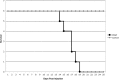
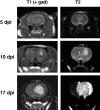
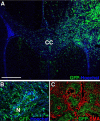
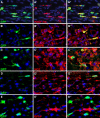
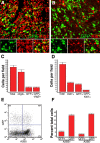
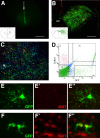
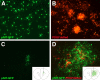
To test the gliomagenic potential of adult glial progenitors, we infected adult rat white matter with a retrovirus that expresses high levels of PDGF and green fluorescent protein (GFP). Tumors that closely resembled human glioblastomas formed in 100% of the animals by 14 d postinfection. Surprisingly, the tumors were composed of a heterogeneous population of cells, <20% of which expressed the retroviral reporter gene (GFP). The vast majority of both GFP+ and GFP- tumor cells expressed markers of glial progenitors. Thus, the tumors arose from the massive expansion of both infected and uninfected glial progenitors, suggesting that PDGF was driving tumor formation via autocrine and paracrine stimulation of glial progenitor cells. To explore this possibility further, we coinjected a retrovirus expressing PDGF-IRES-DsRed with a control retrovirus expressing only GFP. The resulting tumors contained a mixture of red cells (PDGF-expressing/tumor-initiating cells) and green cells (recruited progenitors). Both populations were highly proliferative and infiltrative. In contrast, when the control GFP retrovirus was injected alone, the animals never formed tumors and the majority of infected cells differentiated along the oligodendrocyte lineage. Together, these results reveal that adult white matter progenitors not only have the capacity to give rise to gliomas, but resident progenitors are recruited to proliferate within the mitogenic environment of the tumor and in this way contribute significantly to the heterogeneous mass of cells that compose a malignant glioma.
Figures
References
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA (2002). Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1:269–277. - PubMed
-
- Berkman RA, Clark WC, Saxena A, Robertson JT, Oldfield EH, Ali IU (1992). Clonal composition of glioblastoma multiforme. J Neurosurg 77:432–437. - PubMed
-
- Blankenberg FG, Teplitz RL, Ellis W, Salamat MS, Min BH, Hall L, Boothroyd DB, Johnstone IM, Enzmann DR (1995). The influence of volumetric tumor doubling time, DNA ploidy, and histologic grade on the survival of patients with intracranial astrocytomas. AJNR Am J Neuroradiol 16:1001–1012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
