Linking neural representation to function in stereoscopic depth perception: roles of the middle temporal area in coarse versus fine disparity discrimination
- PMID: 16793886
- PMCID: PMC1994558
- DOI: 10.1523/JNEUROSCI.5435-05.2006
Linking neural representation to function in stereoscopic depth perception: roles of the middle temporal area in coarse versus fine disparity discrimination
Abstract
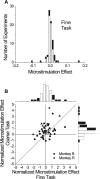
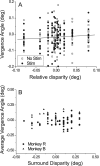
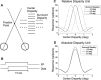
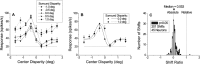
Neurons selective for binocular disparity form the neural substrate for stereoscopic depth perception and are found in several areas of primate visual cortex. Presumably, multiple representations of disparity exist to serve different functions, but the specific contributions of different visual areas to depth perception remain poorly understood. We examine this issue by comparing the contributions of the middle temporal (MT) area to performance of two depth discrimination tasks: a "coarse" task that involves discrimination between absolute disparities in the presence of noise, and a "fine" task that involves discrimination of very small differences in relative disparity between two stimuli in the absence of noise. In the fine task, we find that electrical microstimulation of MT does not affect perceptual decisions, although many individual MT neurons have sufficient sensitivity to account for behavioral performance. In contrast, microstimulation at the same recording sites does bias depth percepts in the coarse task. We hypothesized that these results may be explained by the fact that MT neurons do not represent relative disparity signals that are thought to be essential for the fine task. This hypothesis was supported by single-unit recordings that show that MT neurons signal absolute, but not relative, disparities in a stimulus configuration similar to that used in the fine task. This work establishes a link between the neural representation of disparity in MT and the functional contributions of this area to depth perception.
Figures




Similar articles
-
Contribution of middle temporal area to coarse depth discrimination: comparison of neuronal and psychophysical sensitivity.J Neurosci. 2003 Apr 15;23(8):3515-30. doi: 10.1523/JNEUROSCI.23-08-03515.2003. J Neurosci. 2003. PMID: 12716961 Free PMC article.
-
Decision-Related Activity in Macaque V2 for Fine Disparity Discrimination Is Not Compatible with Optimal Linear Readout.J Neurosci. 2017 Jan 18;37(3):715-725. doi: 10.1523/JNEUROSCI.2445-16.2016. J Neurosci. 2017. PMID: 28100751 Free PMC article.
-
A functional link between MT neurons and depth perception based on motion parallax.J Neurosci. 2015 Feb 11;35(6):2766-77. doi: 10.1523/JNEUROSCI.3134-14.2015. J Neurosci. 2015. PMID: 25673864 Free PMC article.
-
Mechanisms of stereopsis in monkey visual cortex.Cereb Cortex. 1995 May-Jun;5(3):193-204. doi: 10.1093/cercor/5.3.193. Cereb Cortex. 1995. PMID: 7613075 Review.
-
Coarse-fine dichotomies in human stereopsis.Vision Res. 2009 Nov;49(22):2653-65. doi: 10.1016/j.visres.2009.06.004. Epub 2009 Jun 9. Vision Res. 2009. PMID: 19520102 Review.
Cited by
-
Microstructural properties of the vertical occipital fasciculus explain the variability in human stereoacuity.Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12289-12294. doi: 10.1073/pnas.1804741115. Epub 2018 Nov 14. Proc Natl Acad Sci U S A. 2018. PMID: 30429321 Free PMC article.
-
Effects of Microstimulation in the Anterior Intraparietal Area during Three-Dimensional Shape Categorization.PLoS One. 2015 Aug 21;10(8):e0136543. doi: 10.1371/journal.pone.0136543. eCollection 2015. PLoS One. 2015. PMID: 26295941 Free PMC article.
-
Clinical features of acute acquired comitant esotropia in the Chinese populations.Medicine (Baltimore). 2017 Nov;96(46):e8528. doi: 10.1097/MD.0000000000008528. Medicine (Baltimore). 2017. PMID: 29145257 Free PMC article.
-
The role of binocular disparity and attention in the neural representation of multiple moving stimuli in the visual cortex.bioRxiv [Preprint]. 2024 Sep 19:2023.06.25.546480. doi: 10.1101/2023.06.25.546480. bioRxiv. 2024. PMID: 37425944 Free PMC article. Preprint.
-
Fine discrimination training alters the causal contribution of macaque area MT to depth perception.Neuron. 2008 Oct 23;60(2):367-77. doi: 10.1016/j.neuron.2008.08.023. Neuron. 2008. PMID: 18957227 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
