Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival
- PMID: 16793887
- PMCID: PMC6673824
- DOI: 10.1523/JNEUROSCI.0526-06.2006
Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival
Abstract
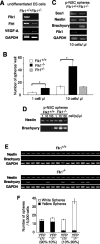
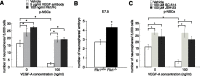
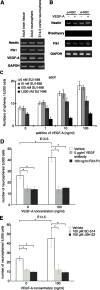
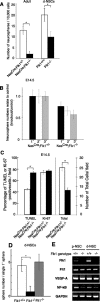
There are two types of neural stem cells (NSCs). Primitive NSCs [leukemia inhibitory factor (LIF) dependent but exogenous fibroblast growth factor (FGF) 2 independent] can be derived from mouse embryonic stem (ES) cells in vitro and from embryonic day 5.5 (E5.5) to E7.5 epiblast and E7.5-E8.5 neuroectoderm in vivo. Definitive NSCs (LIF independent but FGF2 dependent) first appear in the E8.5 neural plate and persist throughout life. Primitive NSCs give rise to definitive NSCs. Loss and gain of functions were used to study the role of vascular endothelial growth factor (VEGF)-A and its receptor, Flk1, in NSCs. The numbers of Flk1 knock-out mice embryo-derived and ES cell-derived primitive NSCs were increased because of the enhanced survival of primitive NSCs. In contrast, neural precursor-specific, Flk1 conditional knock-out mice-derived, definitive NSCs numbers were decreased because of the enhanced cell death of definitive NSCs. These effects were not observed in cells lacking Flt1, another VEGF receptor. In addition, the cell death stimulated by VEGF-A of primitive NSC and the cell survival stimulated by VEGF-A of definitive NSC were blocked by Flk1/Fc-soluble receptors and VEGF-A function-blocking antibodies. These VEGF-A phenotypes also were blocked by inhibition of the downstream effector nuclear factor kappaB (NF-kappaB). Thus, both the cell death of primitive NSC and the cell survival of definitive NSC induced by VEGF-A stimulation are mediated by bifunctional NF-kappaB effects. In conclusion, VEGF-A function through Flk1 mediates survival (and not proliferative or fate change) effects on NSCs, specifically.
Figures

Similar articles
-
Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling.Genes Dev. 2004 Aug 1;18(15):1806-11. doi: 10.1101/gad.1208404. Genes Dev. 2004. PMID: 15289455 Free PMC article.
-
Homologous transplantation of neural stem cells to the injured spinal cord of mice.Neurosurgery. 2005 Nov;57(5):1014-25; discussion 1014-25. doi: 10.1227/01.neu.0000180058.58372.4c. Neurosurgery. 2005. PMID: 16284571
-
Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia.Neurosurgery. 2005 Aug;57(2):325-33; discussion 325-33. doi: 10.1227/01.neu.0000166682.50272.bc. Neurosurgery. 2005. PMID: 16094163
-
[The generation of neural stem cells: induction of neural stem cells from embryonic stem (ES) cells].Rinsho Shinkeigaku. 2003 Nov;43(11):827-9. Rinsho Shinkeigaku. 2003. PMID: 15152476 Review. Japanese.
-
Signal transduction pathways involved in the lineage-differentiation of NSCs: can the knowledge gained from blood be used in the brain?Cancer Invest. 2004;22(6):925-43. doi: 10.1081/cnv-200039679. Cancer Invest. 2004. PMID: 15641490 Review.
Cited by
-
Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment.PLoS One. 2007 Apr 18;2(4):e373. doi: 10.1371/journal.pone.0000373. PLoS One. 2007. PMID: 17440609 Free PMC article.
-
Proliferation but not migration is associated with blood vessels during development of the rostral migratory stream.Dev Neurosci. 2010 Aug;32(3):163-72. doi: 10.1159/000301135. Epub 2010 Jul 10. Dev Neurosci. 2010. PMID: 20616553 Free PMC article.
-
Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression.Behav Brain Res. 2012 Feb 14;227(2):440-9. doi: 10.1016/j.bbr.2011.04.022. Epub 2011 Apr 22. Behav Brain Res. 2012. PMID: 21536078 Free PMC article. Review.
-
Molecular mediators of angiogenesis and neurogenesis after ischemic stroke.Rev Neurosci. 2022 Sep 8;34(4):425-442. doi: 10.1515/revneuro-2022-0049. Print 2023 Jun 27. Rev Neurosci. 2022. PMID: 36073599 Free PMC article.
-
VEGF ligands and receptors: implications in neurodevelopment and neurodegeneration.Cell Mol Life Sci. 2013 May;70(10):1763-78. doi: 10.1007/s00018-013-1283-7. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23475071 Free PMC article. Review.
References
-
- Barkett M, Gilmore TD (1999). Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18:6910–6924. - PubMed
-
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL (1999). Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283:534–537. - PubMed
-
- Boguslawski G, McGlynn PW, Harvey KA, Kovala AT (2004). SU1498, an inhibitor of vascular endothelial growth factor receptor 2, causes accumulation of phosphorylated ERK kinases and inhibits their activity in vivo and in vitro. J Biol Chem 279:5716–5724. - PubMed
-
- Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M (2005). A novel role for VEGF as an autocrine survival factor for embryonic stem cells during hypoxia. J Biol Chem 280:3493–3499. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
