Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats
- PMID: 16793907
- PMCID: PMC1819447
- DOI: 10.1113/jphysiol.2006.105106
Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats
Abstract
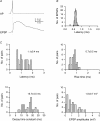
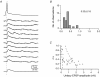
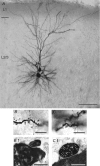
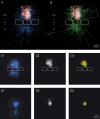
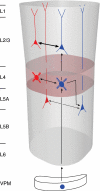
Synaptically coupled layer 2/3 (L2/3) pyramidal neurones located above the same layer 4 barrel ('barrel-related') were investigated using dual whole-cell voltage recordings in acute slices of rat somatosensory cortex. Recordings were followed by reconstructions of biocytin-filled neurones. The onset latency of unitary EPSPs was 1.1 +/- 0.4 ms, the 20-80% rise time was 0.7 +/- 0.2 ms, the average amplitude was 1.0 +/- 0.7 mV and the decay time constant was 15.7 +/- 4.5 ms. The coefficient of variation (c.v.) of unitary EPSP amplitudes decreased with increasing EPSP peak and was 0.33 +/- 0.18. Bursts of APs in the presynaptic pyramidal cell resulted in EPSPs that, over a wide range of frequencies (5-100 Hz), displayed amplitude depression. Anatomically the barrel-related pyramidal cells in the lower half of layer 2/3 have a long apical dendrite with a small terminal tuft, while pyramidal cells in the upper half of layer 2/3 have shorter and often more 'irregularly' shaped apical dendrites that branch profusely in layer 1. The number of putative excitatory synaptic contacts established by the axonal collaterals of a L2/3 pyramidal cell with a postsynaptic pyramidal cell in the same column varied between 2 and 4, with an average of 2.8 +/- 0.7 (n = 8 pairs). Synaptic contacts were established predominantly on the basal dendrites at a mean geometric distance of 91 +/- 47 mum from the pyramidal cell soma. L2/3-to-L2/3 connections formed a blob-like innervation domain containing 2.8 mm of the presynaptic axon collaterals with a bouton density of 0.3 boutons per mum axon. Within the supragranular layers of its home column a single L2/3 pyramidal cell established about 900 boutons suggesting that 270 pyramidal cells in layer 2/3 are innervated by an individual pyramidal cell. In turn, a single pyramidal cell received synaptic inputs from 270 other L2/3 pyramidal cells. The innervation domain of L2/3-to-L2/3 connections superimposes almost exactly with that of L4-to-L2/3 connections. This suggests that synchronous feed-forward excitation of L2/3 pyramidal cells arriving from layer 4 could be potentially amplified in layer 2/3 by feedback excitation within a column and then relayed to the neighbouring columns.
Figures






References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neurosci. 1991;41:365–379. - PubMed
-
- Armstrong-James M. The nature and plasticity of sensory processing within adult rat barrel cortex. In: Jones EG, Diamond IT, editors. The Barrel Cortex of Rodents. New York: Plenum Press; 1995. pp. 333–374.
-
- Armstrong-James M, Callahan CA, Friedman MA. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991;303:193–210. - PubMed
-
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 ‘barrel’ cortex. J Comp Neurol. 1987;263:265–281. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

