Chronic ethanol feeding suppresses beta-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat
- PMID: 16794014
- PMCID: PMC1764504
- DOI: 10.1210/en.2006-0120
Chronic ethanol feeding suppresses beta-adrenergic receptor-stimulated lipolysis in adipocytes isolated from epididymal fat
Abstract
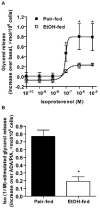
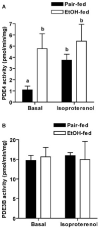
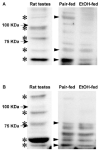
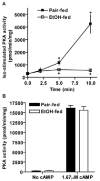
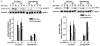
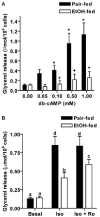
Chronic ethanol consumption disrupts G protein-dependent signaling pathways in rat adipocytes. Because lipolysis in adipocytes is regulated by G protein-mediated cAMP signal transduction, we hypothesized that cAMP-regulated lipolysis may be vulnerable to long-term ethanol exposure. Male Wistar rats were fed a liquid diet containing ethanol as 35% of total calories or pair-fed a control diet that isocalorically substituted maltose dextrins for ethanol for 4 wk. Lipolysis was measured by glycerol release over 1 h with or without agonists in adipocytes isolated from epididymal fat. Chronic ethanol feeding decreased beta-adrenergic receptor-stimulated lipolysis, but had no effect on basal lipolysis. In response to beta-adrenergic activation, the early peak of cAMP accumulation was suppressed after ethanol feeding, although the basal cAMP concentration in adipocytes did not differ between pair- and ethanol-fed rats. The suppression in cAMP accumulation caused by ethanol feeding was associated with increased activity of phosphodiesterase 4. Chronic ethanol feeding also decreased beta-adrenergic receptor-stimulated protein kinase A activation and phosphorylation of its downstream proteins, perilipin A and hormone-sensitive lipase, the primary lipase-mediating lipolysis. In conclusion, these data suggest that chronic ethanol feeding increased phosphodiesterase 4 activity in adipocytes, resulting in decreased accumulation of cAMP in response to beta-adrenergic activation and a suppression of beta-adrenergic stimulation of lipolysis.
Figures



Similar articles
-
Mechanisms of metformin inhibiting lipolytic response to isoproterenol in primary rat adipocytes.J Mol Endocrinol. 2009 Jan;42(1):57-66. doi: 10.1677/JME-08-0130. Epub 2008 Oct 27. J Mol Endocrinol. 2009. PMID: 18955435
-
Medium-chain Fatty acids attenuate agonist-stimulated lipolysis, mimicking the effects of starvation.Obes Res. 2004 Apr;12(4):599-611. doi: 10.1038/oby.2004.69. Obes Res. 2004. PMID: 15090627
-
Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes.Biochim Biophys Acta. 2011 Apr;1812(4):527-35. doi: 10.1016/j.bbadis.2010.10.001. Epub 2010 Oct 19. Biochim Biophys Acta. 2011. PMID: 20969954
-
Regulation of adipocyte lipolysis.Nutr Res Rev. 2014 Jun;27(1):63-93. doi: 10.1017/S095442241400002X. Epub 2014 May 28. Nutr Res Rev. 2014. PMID: 24872083 Review.
-
The central role of perilipin a in lipid metabolism and adipocyte lipolysis.IUBMB Life. 2004 Jul;56(7):379-85. doi: 10.1080/15216540400009968. IUBMB Life. 2004. PMID: 15545214 Review.
Cited by
-
Adipose inflammation and macrophage infiltration after binge ethanol and burn injury.Alcohol Clin Exp Res. 2014 Jan;38(1):204-13. doi: 10.1111/acer.12210. Epub 2013 Aug 1. Alcohol Clin Exp Res. 2014. PMID: 23909743 Free PMC article.
-
Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway.Mol Cell Biol. 2010 Nov;30(21):5009-20. doi: 10.1128/MCB.00797-10. Epub 2010 Aug 23. Mol Cell Biol. 2010. PMID: 20733001 Free PMC article.
-
Alcohol, White Adipose Tissue, and Brown Adipose Tissue: Mechanistic Links to Lipogenesis and Lipolysis.Nutrients. 2023 Jun 29;15(13):2953. doi: 10.3390/nu15132953. Nutrients. 2023. PMID: 37447280 Free PMC article. Review.
-
Gut Microbiota: Target for Modulation of Gut-Liver-Adipose Tissue Axis in Ethanol-Induced Liver Disease.Mediators Inflamm. 2022 May 20;2022:4230599. doi: 10.1155/2022/4230599. eCollection 2022. Mediators Inflamm. 2022. PMID: 35633655 Free PMC article. Review.
-
Alcohol, Adipose Tissue and Lipid Dysregulation.Biomolecules. 2017 Feb 16;7(1):16. doi: 10.3390/biom7010016. Biomolecules. 2017. PMID: 28212318 Free PMC article.
References
-
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. - PubMed
-
- Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res. 1979;20:289–315. - PubMed
-
- Balasubramaniyan V, Nalini N. The potential beneficial effect of leptin on an experimental model of hyperlipidemia, induced by chronic ethanol treatment. Clin Chim Acta. 2003;337:85–91. - PubMed
-
- Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. - PubMed
-
- Nakamura MT, Cheon Y, Li Y, Nara TY. Mechanisms of regulation of gene expression by fatty acids. Lipids. 2004;39:1077–1083. - PubMed

