Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells
- PMID: 16794260
- PMCID: PMC2643292
- DOI: 10.1165/rcmb.2006-0040OC
Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells
Abstract
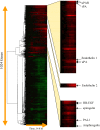
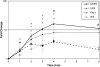
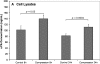
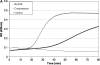
Mechanical stimulation of the airway epithelium, as would occur during bronchoconstriction, is a potent stimulus and can activate profibrotic pathways. We used DNA microarray technology to examine gene expression in compressed normal human bronchial epithelial cells (NHBE). Compressive stress applied continuously over an 8-h period to NHBE cells led to the upregulation of several families of genes, including a family of plasminogen-related genes that were previously not known to be regulated in this system. Real-time PCR demonstrated a peak increase in gene expression of 8.0-fold for urokinase plasminogen activator (uPA), 16.2-fold for urokinase plasminogen activator receptor (uPAR), 4.2-fold for plasminogen activator inhibitor-1 (PAI-1), and 3.9-fold for tissue plasminogen activator (tPA). Compressive stress also increased uPA protein levels in the cell lysates (112.0 versus 82.0 ng/ml, P = 0.0004), and increased uPA (4.7 versus 3.3 ng/ml, P = 0.02), uPAR (1.3 versus 0.86 ng/ml, P = 0.007), and PAI-1 (50 versus 36 ng/ml, P = 0.006) protein levels in cell culture media. Functional studies demonstrated increased urokinase-dependent plasmin generation in compression-stimulated cells (0.0090 versus 0.0033 OD/min, P = 0.03). In addition, compression led to increased activation of matrix metalloproteinase (MMP)-9 and MMP-2 in a urokinase-dependent manner. In postmortem human lung tissue, we observed an increase in epithelial uPA and uPAR immunostaining in the airways of two patients who died in status asthmaticus compared with minimal immunoreactivity noted in airways from seven lung donors without asthma. Together these observations suggest an integrated response of airway epithelial cells to mechanical stimulation, acting through the plasminogen-activating system to modify the airway microenvironment.
Figures







References
-
- Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol 2003; 111:215–225; quiz 226. - PubMed
-
- Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol 2003;28:142–149. - PubMed
-
- Wiggs BR, Hrousis CA, Drazen JM, Kamm RD. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol 1997;83:1814–1821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

