Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation
- PMID: 16794579
- PMCID: PMC1500971
- DOI: 10.1038/sj.emboj.7601191
Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation
Abstract
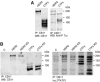
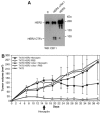
The overactivation of the HERs, a family of tyrosine kinase receptors, leads to the development of cancer. Although the canonical view contemplates HER receptors restricted to the secretory and endocytic pathways, full-length HER1, HER2 and HER3 have been detected in the nucleoplasm. Furthermore, limited proteolysis of HER4 generates nuclear C-terminal fragments (CTFs). Using cells expressing a panel of deletion and point mutants, here we show that HER2 CTFs are generated by alternative initiation of translation from methionines located near the transmembrane domain of the full-length molecule. In vitro and in vivo, HER2 CTFs are found in the cytoplasm and nucleus. Expression of HER2 CTFs to levels similar to those found in human tumors induces the growth of breast cancer xenografts in nude mice. Tumors dependent on CTFs are sensitive to inhibitors of the kinase activity but do not respond to therapeutic antibodies against HER2. Thus, the kinase domain seems necessary for the activity of HER2 CTFs and the presence of these HER2 fragments could account for the resistance to treatment with antibodies.
Figures







References
-
- Arribas J, Borroto A (2002) Protein ectodomain shedding. Chem Rev 102: 4627–4638 - PubMed
-
- Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rosejohn S, Massagué J (1996) Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem 271: 11376–11382 - PubMed
-
- Bagatell R, Khan O, Paine-Murrieta G, Taylor CW, Akinaga S, Whitesell L (2001) Destabilization of steroid receptors by heat shock protein 90-binding drugs: a ligand-independent approach to hormonal therapy of breast cancer. Clin Cancer Res 7: 2076–2084 - PubMed
-
- Baselga J, Norton L (2002) Focus on breast cancer. Cancer Cell 1: 319–322 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

