Heat shock inhibits both aminoglycoside- and cisplatin-induced sensory hair cell death
- PMID: 16794914
- PMCID: PMC2504613
- DOI: 10.1007/s10162-006-0043-x
Heat shock inhibits both aminoglycoside- and cisplatin-induced sensory hair cell death
Abstract
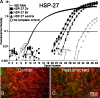
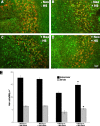
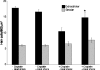
Human hearing and balance impairments are often attributable to the death of sensory hair cells in the inner ear. These cells are hypersensitive to death induced by noise exposure, aging, and some therapeutic drugs. Two major classes of ototoxic drugs are the aminoglycoside antibiotics and the antineoplastic agent cisplatin. Exposure to these drugs leads to hair cell death that is mediated by the activation of specific apoptotic proteins, including caspases. The induction of heat shock proteins (HSPs) in response to cellular stress is a ubiquitous and highly conserved response that can significantly inhibit apoptosis in some systems by inhibiting apoptotic proteins. Induction of HSPs occurs in hair cells in response to a variety of stimuli. Given that HSPs can directly inhibit apoptosis, we hypothesized that heat shock may inhibit apoptosis in hair cells exposed to ototoxic drugs. To test this hypothesis, we developed a method for inducing HSP expression in the adult mouse utricle in vitro. In vitro heat shock reliably produces a robust up-regulation of HSP-70 mRNA and protein, as well as more modest up-regulation of HSP-90 and HSP-27. The heat shock does not result in death of hair cells. Heat shock has a significant protective effect against both aminoglycoside- and cisplatin-induced hair cell death in the utricle preparation in vitro. These data indicate that heat shock can inhibit ototoxic drug-induced hair cell death, and that the utricle preparation can be used to examine the molecular mechanism(s) underlying this protective effect.
Figures



References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15782448', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15782448/'}]}
- Adams P, Hendershot G, Marano M. (1999) Current Estimates from the National Health Interview Survey, 1996. National Center for Health Statistics, Hyattsville, MD. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1126/science.3175623', 'is_inner': False, 'url': 'https://doi.org/10.1126/science.3175623'}, {'type': 'PubMed', 'value': '3175623', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/3175623/'}]}
- Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science 241:1817–1820, 1988. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/35019501', 'is_inner': False, 'url': 'https://doi.org/10.1038/35019501'}, {'type': 'PubMed', 'value': '10934466', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10934466/'}]}
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469–475, 2000. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1089/10430340050207948', 'is_inner': False, 'url': 'https://doi.org/10.1089/10430340050207948'}, {'type': 'PubMed', 'value': '11119417', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11119417/'}]}
- Braiden V, Ohtsuru A, Kawashita Y, Miki F, Sawada T, Ito M, Cao Y, Kaneda Y, Koji T, Yamashita S. Eradication of breast cancer xenografts by hyperthermic suicide gene therapy under the control of the heat shock protein promoter. Hum. Gene. Ther. 11:2453–2463, 2000. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/35023595', 'is_inner': False, 'url': 'https://doi.org/10.1038/35023595'}, {'type': 'PubMed', 'value': '10980706', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10980706/'}]}
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2:645–652, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

