Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue
- PMID: 16795045
- PMCID: PMC4048742
- DOI: 10.1002/jcb.20904
Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue
Abstract
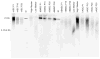
The biologic characteristics of mesenchymal stem cells (MSCs) isolated from two distinct tissues, bone marrow and adipose tissue were evaluated in these studies. MSCs derived from human and non-human primate (rhesus monkey) tissue sources were compared. The data indicate that MSCs isolated from rhesus bone marrow (rBMSCs) and human adipose tissue (hASCs) had more similar biologic properties than MSCs of rhesus adipose tissue (rASCs) and human bone marrow MSCs (hBMSCs). Analyses of in vitro growth kinetics revealed shorter doubling time for rBMSCs and hASCs. rBMSCs and hASCs underwent significantly more population doublings than the other MSCs. MSCs from all sources showed a marked decrease in telomerase activity over extended culture; however, they maintained their mean telomere length. All of the MSCs expressed embryonic stem cell markers, Oct-4, Rex-1, and Sox-2 for at least 10 passages. Early populations of MSCs types showed similar multilineage differentiation capability. However, only the rBMSCs and hASCs retain greater differentiation efficiency at higher passages. Overall in vitro characterization of MSCs from these two species and tissue sources revealed a high level of common biologic properties. However, the results demonstrate clear biologic distinctions, as well.
2006 Wiley-Liss, Inc.
Figures


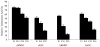



References
-
- Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. - PubMed
-
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. - PubMed
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

