Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids
- PMID: 16796672
- PMCID: PMC5614446
- DOI: 10.1111/j.1365-2958.2006.05177.x
Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids
Erratum in
- Mol Microbiol. 2006 Aug;61(3):838
Abstract
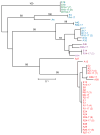
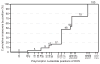
The relapsing fever agent Borrelia hermsii undergoes multiphasic antigenic variation through gene conversion of a unique expression site on a linear plasmid by an archived variable antigen gene. To further characterize this mechanism we assessed the repertoire and organization of archived variable antigen genes by sequencing approximately 85% of plasmids bearing these genes. Most archived genes shared with the expressed gene a <or= 62 nucleotide (nt) region, the upstream homology sequence (UHS), that surrounded the start codon. The 59 archived variable antigen genes were arrayed in clusters with 13 repetitive, 214 nt long downstream homology sequence (DHS) elements distributed among them. A fourteenth DHS element was downstream of the expression locus. Informative nucleotide polymorphisms in UHS regions and DHS elements were applied to the analysis of the expression site of relapse serotypes from 60 infected mice in a prospective study. For most recombinations, the upstream crossover occurred in the UHS's second half, and the downstream crossover was in the DHS's second half. Usually the closest archival DHS element was used, but occasionally a more distant DHS was employed. The downstream extragenic crossover site in B. hermsii contrasts with the upstream [corrected] extragenic crossover site for antigenic variation in African trypanosomes.
Figures


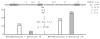


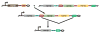
Comment in
-
Antigenic variation with a twist--the Borrelia story.Mol Microbiol. 2006 Jun;60(6):1319-22. doi: 10.1111/j.1365-2958.2006.05204.x. Mol Microbiol. 2006. PMID: 16796669
References
-
- Aline RF, Jr, Stuart K. Trypanosoma brucei: conserved sequence organization 3′ to telomeric variant surface glycoprotein genes. Exp Parasitol. 1989;68:57–66. - PubMed
-
- Barbour AG. Antigenic variation by relapsing fever Borrelia species and other bacterial pathogens. In: Craig NL, Craigie R, Gellert M, Lambowitz A, editors. Mobile DNA II. Washington, D.C: ASM Press; 2002. pp. 972–994.
-
- Barbour AG, Burman N, Carter CJ, Kitten T, Bergström S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991;5:489–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

