General and specific functions of exonic splicing silencers in splicing control
- PMID: 16797197
- PMCID: PMC1839040
- DOI: 10.1016/j.molcel.2006.05.018
General and specific functions of exonic splicing silencers in splicing control
Abstract
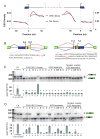
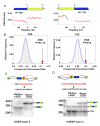
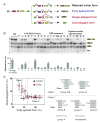
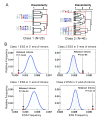
Correct splice site recognition is critical in pre-mRNA splicing. We find that almost all of a diverse panel of exonic splicing silencer (ESS) elements alter splice site choice when placed between competing sites, consistently inhibiting use of intron-proximal 5' and 3' splice sites. Supporting a general role for ESSs in splice site definition, we found that ESSs are both abundant and highly conserved between alternative splice site pairs and that mutation of ESSs located between natural alternative splice site pairs consistently shifted splicing toward the intron-proximal site. Some exonic splicing enhancers (ESEs) promoted use of intron-proximal 5' splice sites, and tethering of hnRNP A1 and SF2/ASF proteins between competing splice sites mimicked the effects of ESS and ESE elements, respectively. Further, we observed that specific subsets of ESSs had distinct effects on a multifunctional intron retention reporter and that one of these subsets is likely preferred for regulation of endogenous intron retention events. Together, our findings provide a comprehensive picture of the functions of ESSs in the control of diverse types of splicing decisions.
Figures

Comment in
-
Unweaving the meanings of messenger RNA sequences.Mol Cell. 2006 Jul 21;23(2):150-1. doi: 10.1016/j.molcel.2006.07.003. Mol Cell. 2006. PMID: 16857580 Review.
References
-
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. - PubMed
-
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

