TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis
- PMID: 16798876
- PMCID: PMC1502469
- DOI: 10.1073/pnas.0604008103
TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis
Abstract
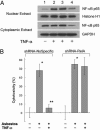
Asbestos is the main cause of human malignant mesothelioma (MM). In vivo, macrophages phagocytize asbestos and, in response, release TNF-alpha and other cytokines that contribute to carcinogenesis through unknown mechanisms. In vitro, asbestos does not induce transformation of primary human mesothelial cells (HM); instead, asbestos is very cytotoxic to HM, causing extensive cell death. This finding raised an apparent paradox: How can asbestos cause MM if HM exposed to asbestos die? We found that asbestos induced the secretion of TNF-alpha and the expression of TNF-alpha receptor I in HM. Treatment of HM with TNF-alpha significantly reduced asbestos cytotoxicity. Through numerous technical approaches, including chemical inhibitors and small interfering RNA strategies, we demonstrate that, in HM, TNF-alpha activates NF-kappaB and that NF-kappaB activation leads to HM survival and resistance to the cytotoxic effects of asbestos. Our data show a critical role for TNF-alpha and NF-kappaB signaling in mediating HM responses to asbestos. TNF-alpha signaling through NF-kappaB-dependent mechanisms increases the percent of HM that survives asbestos exposure, thus increasing the pool of asbestos-damaged HM that are susceptible to malignant transformation. Cytogenetics supported this hypothesis, showing only rare, aberrant metaphases in HM exposed to asbestos and an increased mitotic rate with fewer irregular metaphases in HM exposed to both TNF-alpha and asbestos. Our findings provide a mechanistic rationale for the paradoxical inability of asbestos to transform HM in vitro, elucidate and underscore the role of TNF-alpha in asbestos pathogenesis in humans, and identify potential molecular targets for anti-MM prevention and therapy.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


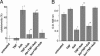



References
-
- Mossman B. T., Bignon J., Corn M., Seaton A., Gee J. B. Science. 1990;247:294–301. - PubMed
-
- Upadhyay D., Kamp D. W. Exp. Biol. Med. (Maywood, NJ) 2003;228:650–659. - PubMed
-
- Carbone M., Kratzke R. A., Testa J. R. Semin. Oncol. 2002;29:2–17. - PubMed
-
- Pass H. I., Hahn S. M., Vogelzang N. J., Carbone M. In: Cancer Principles & Practice of Oncology. 7th Ed. Pass H. I., Vogelzang N., Carbone M., editors. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 1687–1715.
-
- Hesterberg T. W., Butterick C. J., Oshimura M., Brody A. R., Barrett J. C. Cancer Res. 1986;46:5795–5802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

