Exceptional chemical and thermal stability of zeolitic imidazolate frameworks
- PMID: 16798880
- PMCID: PMC1502432
- DOI: 10.1073/pnas.0602439103
Exceptional chemical and thermal stability of zeolitic imidazolate frameworks
Abstract
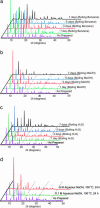
Twelve zeolitic imidazolate frameworks (ZIFs; termed ZIF-1 to -12) have been synthesized as crystals by copolymerization of either Zn(II) (ZIF-1 to -4, -6 to -8, and -10 to -11) or Co(II) (ZIF-9 and -12) with imidazolate-type links. The ZIF crystal structures are based on the nets of seven distinct aluminosilicate zeolites: tetrahedral Si(Al) and the bridging O are replaced with transition metal ion and imidazolate link, respectively. In addition, one example of mixed-coordination imidazolate of Zn(II) and In(III) (ZIF-5) based on the garnet net is reported. Study of the gas adsorption and thermal and chemical stability of two prototypical members, ZIF-8 and -11, demonstrated their permanent porosity (Langmuir surface area = 1,810 m(2)/g), high thermal stability (up to 550 degrees C), and remarkable chemical resistance to boiling alkaline water and organic solvents.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Maesen T. L. M., Marcus B. In: Introduction to Zeolite Science and Practice. van Bekkum H., Flanigen E. M., Jacobs P. A., Jansen J. C., editors. Amsterdam: Elsevier; 2001. pp. 1–9.
-
- Baerlocher C., Meier W. M., Olson D. H. Atlas of Zeolite Framework Types. 5th Ed. Amsterdam: Elsevier; 2001.
-
- Sturm M., Brandl F., Engel. D., Hoppe W. Acta Crystallogr. B. 1975;31:2369–2378.
-
- Lehnert R., Seel F. Z. Anorg. Allg. Chem. 1980;464:187–194.
-
- Rettig S. J., Storr A., Summers D. A., Thompson R. C., Trotter J. Can. J. Chem. 1999;77:425–433.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

