Iron deficiency in cyanobacteria causes monomerization of photosystem I trimers and reduces the capacity for state transitions and the effective absorption cross section of photosystem I in vivo
- PMID: 16798943
- PMCID: PMC1533926
- DOI: 10.1104/pp.106.082339
Iron deficiency in cyanobacteria causes monomerization of photosystem I trimers and reduces the capacity for state transitions and the effective absorption cross section of photosystem I in vivo
Abstract
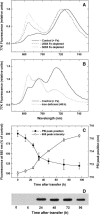
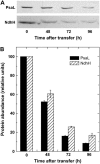
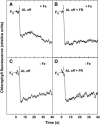
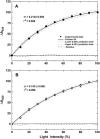
The induction of the isiA (CP43') protein in iron-stressed cyanobacteria is accompanied by the formation of a ring of 18 CP43' proteins around the photosystem I (PSI) trimer and is thought to increase the absorption cross section of PSI within the CP43'-PSI supercomplex. In contrast to these in vitro studies, our in vivo measurements failed to demonstrate any increase of the PSI absorption cross section in two strains (Synechococcus sp. PCC 7942 and Synechocystis sp. PCC 6803) of iron-stressed cells. We report that iron-stressed cells exhibited a reduced capacity for state transitions and limited dark reduction of the plastoquinone pool, which accounts for the increase in PSII-related 685 nm chlorophyll fluorescence under iron deficiency. This was accompanied by lower abundance of the NADP-dehydrogenase complex and the PSI-associated subunit PsaL, as well as a reduced amount of phosphatidylglycerol. Nondenaturating polyacrylamide gel electrophoresis separation of the chlorophyll-protein complexes indicated that the monomeric form of PSI is favored over the trimeric form of PSI under iron stress. Thus, we demonstrate that the induction of CP43' does not increase the PSI functional absorption cross section of whole cells in vivo, but rather, induces monomerization of PSI trimers and reduces the capacity for state transitions. We discuss the role of CP43' as an effective energy quencher to photoprotect PSII and PSI under unfavorable environmental conditions in cyanobacteria in vivo.
Figures



Similar articles
-
The induction of CP43' by iron-stress in Synechococcus sp. PCC 7942 is associated with carotenoid accumulation and enhanced fatty acid unsaturation.Biochim Biophys Acta. 2007 Jun;1767(6):807-13. doi: 10.1016/j.bbabio.2007.02.006. Epub 2007 Feb 13. Biochim Biophys Acta. 2007. PMID: 17362874
-
Time-resolved absorption and emission show that the CP43' antenna ring of iron-stressed synechocystis sp. PCC6803 is efficiently coupled to the photosystem I reaction center core.Biochemistry. 2003 Apr 8;42(13):3893-903. doi: 10.1021/bi026987u. Biochemistry. 2003. PMID: 12667080
-
Spectroscopic study of the CP43' complex and the PSI-CP43' supercomplex of the cyanobacterium Synechocystis PCC 6803.J Phys Chem B. 2011 Nov 17;115(45):13339-49. doi: 10.1021/jp206054b. Epub 2011 Oct 26. J Phys Chem B. 2011. PMID: 21978372
-
Structure and functional role of supercomplexes of IsiA and Photosystem I in cyanobacterial photosynthesis.FEBS Lett. 2005 Jun 13;579(15):3253-7. doi: 10.1016/j.febslet.2005.03.051. Epub 2005 Apr 7. FEBS Lett. 2005. PMID: 15943969 Review.
-
Light harvesting in photosystem I supercomplexes.Biochemistry. 2006 Jan 17;45(2):331-45. doi: 10.1021/bi051932o. Biochemistry. 2006. PMID: 16401064 Review.
Cited by
-
Unveiling large charge transfer character of PSII in an iron-deficient cyanobacterial membrane: A Stark fluorescence spectroscopy study.Photosynth Res. 2024 Jun;160(2-3):77-86. doi: 10.1007/s11120-024-01099-1. Epub 2024 Apr 15. Photosynth Res. 2024. PMID: 38619701
-
Association of small CAB-like proteins (SCPs) of Synechocystis sp. PCC 6803 with Photosystem II.Photosynth Res. 2008 Feb-Mar;95(2-3):135-45. doi: 10.1007/s11120-007-9244-3. Epub 2007 Oct 3. Photosynth Res. 2008. PMID: 17912610
-
Characterization and evolution of tetrameric photosystem I from the thermophilic cyanobacterium Chroococcidiopsis sp TS-821.Plant Cell. 2014 Mar;26(3):1230-45. doi: 10.1105/tpc.113.120782. Epub 2014 Mar 28. Plant Cell. 2014. PMID: 24681621 Free PMC article.
-
Structural basis for energy and electron transfer of the photosystem I-IsiA-flavodoxin supercomplex.Nat Plants. 2020 Feb;6(2):167-176. doi: 10.1038/s41477-020-0593-7. Epub 2020 Feb 10. Nat Plants. 2020. PMID: 32042157
-
Functional Connectivity of Red Chlorophylls in Cyanobacterial Photosystem I Revealed by Fluence-Dependent Transient Absorption.J Phys Chem B. 2025 Mar 27;129(12):3191-3197. doi: 10.1021/acs.jpcb.5c00198. Epub 2025 Mar 18. J Phys Chem B. 2025. PMID: 40100810 Free PMC article.
References
-
- Andrizhiyevskaya EG, Schwabe TME, Germano M, D'Haene S, Kruip J, van Grondelle R, Dekker JP (2002) Spectroscopic properties of PSI-IsiA supercomplex from the cyanobacterium Synechococcus PCC 7942. Biochim Biophys Acta 1556: 265–272 - PubMed
-
- Ardelean I, Matthijs HCP, Havaux M, Joset F, Jeanjean R (2002) Unexpected changes in photosystem I function in a cytochrome c6-deficient mutant of the cyanobacterium Synechocystis PCC 6803. FEMS Microbiol Lett 213: 113–119 - PubMed
-
- Asada K, Heber U, Schreiber U (1993) Electron flow to the intersystem chain from stromal components and cyclic electron flow in maize chloroplasts, as determined in intact leaves by monitoring redox change of P700 and chlorophyll fluorescence. Plant Cell Physiol 34: 39–50
-
- Aspinwall CL, Duncan J, Bibby T, Mullineaux CW, Barber J (2004) The trimeric organization of photosystem I is not necessary for the iron-stress induced CP43′ protein to functionally associate with this reaction centre. FEBS Lett 574: 126–130 - PubMed
-
- Bibby TS, Nield J, Barber J (2001. a) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412: 743–745 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

