ORC binding to TRF2 stimulates OriP replication
- PMID: 16799465
- PMCID: PMC1500828
- DOI: 10.1038/sj.embor.7400730
ORC binding to TRF2 stimulates OriP replication
Abstract
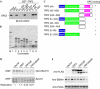
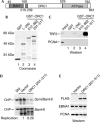
In higher eukaryotes, the origin recognition complex (ORC) lacks sequence-specific DNA binding, and it remains unclear what other factors specify an origin of DNA replication. The Epstein-Barr virus origin of plasmid replication (OriP) recruits ORC, but the precise mechanism of ORC recruitment and origin activation is not clear. We now show that ORC is recruited selectively to the dyad symmetry (DS) region of OriP as a consequence of direct interactions with telomere repeat factor 2 (TRF2) and ORC1. TRF-binding sites within DS stimulate replication initiation and facilitate ORC recruitment in vitro and in vivo. TRF2, but not TRF1 or hRap1, recruits ORC from nuclear extracts. The amino-terminal domain of TRF2 associated with a specific region of ORC1 and was necessary for stimulation of DNA replication. These results support a model in which TRF2 stimulates OriP replication activity by direct binding with ORC subunits.
Figures



References
-
- Atanasiu C, Lezina L, Lieberman PM (2005) DNA affinity purification of Epstein–Barr virus OriP-binding proteins. Methods Mol Biol 292: 267–276 - PubMed
-
- Beall EL, Manak JR, Zhou S, Bell M, Lipsick JS, Botchan MR (2002) Role for a Drosophila Myb-containing protein complex in site-specific DNA replication. Nature 420: 833–837 - PubMed
-
- Bell SP (2002) The origin recognition complex: from simple origins to complex functions. Genes Dev 16: 659–672 - PubMed
-
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

