Ancient evolutionary origin of diversified variable regions demonstrated by crystal structures of an immune-type receptor in amphioxus
- PMID: 16799561
- PMCID: PMC3707131
- DOI: 10.1038/ni1359
Ancient evolutionary origin of diversified variable regions demonstrated by crystal structures of an immune-type receptor in amphioxus
Abstract
Although the origins of genes encoding the rearranging binding receptors remain obscure, it is predicted that their ancestral forms were nonrearranging immunoglobulin-type domains. Variable region-containing chitin-binding proteins (VCBPs) are diversified immune-type molecules found in amphioxus (Branchiostoma floridae), an invertebrate that diverged early in deuterostome phylogeny. To study the potential evolutionary relationships between VCBPs and vertebrate adaptive immune receptors, we solved the structures of both a single V-type domain (to 1.15 A) and a pair of V-type domains (to 1.85 A) from VCBP3. The deduced structures show integral features of the ancestral variable-region fold as well as unique features of variable-region pairing in molecules that may reflect characteristics of ancestral forms of diversified immune receptors found in modern-day vertebrates.
Figures
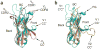


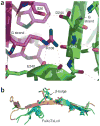

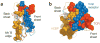
Comment in
-
Recognition reversal in a spineless scrounger.Nat Immunol. 2006 Aug;7(8):797-8. doi: 10.1038/ni0806-797. Nat Immunol. 2006. PMID: 16855603 No abstract available.
References
-
- Orengo CA, Thornton JM. Protein families and their evolution—a structural perspective. Annu Rev Biochem. 2005;74:867–900. - PubMed
-
- Bork P, Holm L, Sander C. The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol. 1994;242:309–320. - PubMed
-
- Barclay AN. Membrane proteins with immunoglobulin-like domains–a master super-family of interaction molecules. Semin Immunol. 2003;15:215–223. - PubMed
-
- Hunkapiller T, Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–62. - PubMed
-
- Eason DD, et al. Mechanisms of antigen receptor evolution. Semin Immunol. 2004;16:215–226. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

