Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from a transgenic rat expressing alkaline phosphatase marker gene
- PMID: 16799702
- PMCID: PMC1482729
- DOI: 10.1017/S1740925X04000079
Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from a transgenic rat expressing alkaline phosphatase marker gene
Abstract
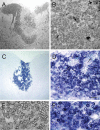
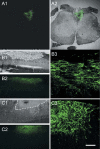
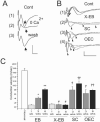
Transplantation of cell suspensions containing olfactory ensheathing cells (OECs) has been reported to remyelinate demyelinated axons in the spinal cord with a Schwann cell (SC)-like pattern of myelination. However, questions have been raised recently as to whether OECs can form SC-like myelin. To address this issue we prepared SCs and OECs from transgenic rats in which a marker gene, human placental alkaline phosphatase (hPAP), is linked to the ubiquitously active promoter of the R26 gene. SCs were prepared from the sciatic nerve and OECs from the outer nerve-fiber layer of the olfactory bulb. Positive S100 and p75 immunostaining indicated that >95% of cells in culture displayed either SC or OEC phenotypes. Suspensions of either SCs or OECs were transplanted into an X-irradiation/ethidium bromide demyelinating lesion in the spinal cord. We observed extensive SC-like remyelination following either SC or OEC transplantation 3 weeks after injection of the cells. Alkaline phosphatase (ALP) chromagen reaction product was associated clearly with the myelin-forming cells. Thus, cell suspensions that are enriched in either SCs or OECs result in peripheral-like myelin when transplanted in vivo.
Figures


References
-
- Au W, Treloar HB, Greer CA. Sublaminar organization of the mouse olfactory bulb nerve layer. Journal of Comparative Neurology. 2002;446:68–80. - PubMed
-
- Au W, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;3:224–236. - PubMed
-
- Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, et al. Identification of a human olfactory ensheathing cell that can effect transplant-medicated remyelination of demyelinated CNS axons. Brain. 2000;123:1581–1588. - PubMed
-
- Baron-Van Evercooren A, Gansmuller A, Duhamel E, Pascal F, Gumpel M. Repair of a myelin lesion by Schwann cells transplanted into the adult mouse spinal cord. Journal of Neuroimunology. 1992;40:235–242. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
