The visible touch: in planta visualization of protein-protein interactions by fluorophore-based methods
- PMID: 16800872
- PMCID: PMC1523328
- DOI: 10.1186/1746-4811-2-12
The visible touch: in planta visualization of protein-protein interactions by fluorophore-based methods
Abstract
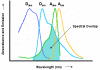
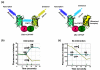
Non-invasive fluorophore-based protein interaction assays like fluorescence resonance energy transfer (FRET) and bimolecular fluorescence complementation (BiFC, also referred to as "split YFP") have been proven invaluable tools to study protein-protein interactions in living cells. Both methods are now frequently used in the plant sciences and are likely to develop into standard techniques for the identification, verification and in-depth analysis of polypeptide interactions. In this review, we address the individual strengths and weaknesses of both approaches and provide an outlook about new directions and possible future developments for both techniques.
Figures




References
-
- The Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. - PubMed
-
- Goff SA, Ricke D, Lan TH, Presting G, Wang RL, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, Hadley D, Hutchinson D, Martin C, Katagiri F, Lange BM, Moughamer T, Xia Y, Budworth P, Zhong JP, Miguel T, Paszkowski U, Zhang SP, Colbert M, Sun WL, Chen LL, Cooper B, Park S, Wood TC, Mao L, Quail P, Wing R, Dean R, Yu YS, Zharkikh A, Shen R, Sahasrabudhe S, Thomas A, Cannings R, Gutin A, Pruss D, Reid J, Tavtigian S, Mitchell J, Eldredge G, Scholl T, Miller RM, Bhatnagar S, Adey N, Rubano T, Tusneem N, Robinson R, Feldhaus J, Macalma T, Oliphant A, Briggs S. A draft sequence of the rice genome (Oryza sativa L. ssp japonica) Science. 2002;296:92–100. - PubMed
-
- Yu J, Hu SN, Wang J, Wong GKS, Li SG, Liu B, Deng YJ, Dai L, Zhou Y, Zhang XQ, Cao ML, Liu J, Sun JD, Tang JB, Chen YJ, Huang XB, Lin W, Ye C, Tong W, Cong LJ, Geng JN, Han YJ, Li L, Li W, Hu GQ, Huang XG, Li WJ, Li J, Liu ZW, Liu JP, Qi QH, Liu JS, Li T, Wang XG, Lu H, Wu TT, Zhu M, Ni PX, Han H, Dong W, Ren XY, Feng XL, Cui P, Li XR, Wang H, Xu X, Zhai WX, Xu Z, Zhang JS, He SJ, Zhang JG, Xu JC, Zhang KL, Zheng XW, Dong JH, Zeng WY, Tao L, Ye J, Tan J, Ren XD, Chen XW, He J, Liu DF, Tian W, Tian CG, Xia HG, Bao QY, Li G, Gao H, Cao T, Zhao WM, Li P, Chen W, Wang XD, Zhang Y, Hu JF, Liu S, Yang J, Zhang GY, Xiong YQ, Li ZJ, Mao L, Zhou CS, Zhu Z, Chen RS, Hao BL, Zheng WM, Chen SY, Guo W, Li GJ, Liu SQ, Tao M, Zhu LH, Yuan LP, Yang HM. A draft sequence of the rice genome (Oryza sativa L. ssp indica) Science. 2002;296:79–92. - PubMed
-
- Causier B, Davies B. Analysing protein-protein interactions with the yeast two-hybrid system. Plant Mol Biol. 2002;50:855–870. - PubMed
-
- Masters MC. Co-immunoprecipitation from transfected cells. Methods Mol Biol. 2004;261:337–350. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

