Mechanism of the inhibition of Ca2+-activated Cl- currents by phosphorylation in pulmonary arterial smooth muscle cells
- PMID: 16801382
- PMCID: PMC2151553
- DOI: 10.1085/jgp.200609507
Mechanism of the inhibition of Ca2+-activated Cl- currents by phosphorylation in pulmonary arterial smooth muscle cells
Abstract
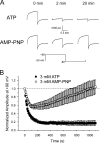
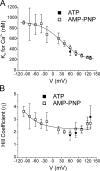
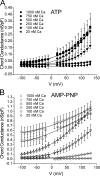
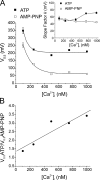
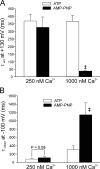
The aim of the present study was to provide a mechanistic insight into how phosphatase activity influences calcium-activated chloride channels in rabbit pulmonary artery myocytes. Calcium-dependent Cl- currents (I(ClCa)) were evoked by pipette solutions containing concentrations between 20 and 1000 nM Ca2+ and the calcium and voltage dependence was determined. Under control conditions with pipette solutions containing ATP and 500 nM Ca2+, I(ClCa) was evoked immediately upon membrane rupture but then exhibited marked rundown to approximately 20% of initial values. In contrast, when phosphorylation was prohibited by using pipette solutions containing adenosine 5'-(beta,gamma-imido)-triphosphate (AMP-PNP) or with ATP omitted, the rundown was severely impaired, and after 20 min dialysis, I(ClCa) was approximately 100% of initial levels. I(ClCa) recorded with AMP-PNP-containing pipette solutions were significantly larger than control currents and had faster kinetics at positive potentials and slower deactivation kinetics at negative potentials. The marked increase in I(ClCa) was due to a negative shift in the voltage dependence of activation and not due to an increase in the apparent binding affinity for Ca2+. Mathematical simulations were carried out based on gating schemes involving voltage-independent binding of three Ca2+, each binding step resulting in channel opening at fixed calcium but progressively greater "on" rates, and voltage-dependent closing steps ("off" rates). Our model reproduced well the Ca2+ and voltage dependence of I(ClCa) as well as its kinetic properties. The impact of global phosphorylation could be well mimicked by alterations in the magnitude, voltage dependence, and state of the gating variable of the channel closure rates. These data reveal that the phosphorylation status of the Ca2+-activated Cl- channel complex influences current generation dramatically through one or more critical voltage-dependent steps.
Figures




Similar articles
-
Differential regulation of Ca(2+)-activated Cl(-) currents in rabbit arterial and portal vein smooth muscle cells by Ca(2+)-calmodulin-dependent kinase.J Physiol. 2001 Jul 15;534(Pt. 2):395-408. doi: 10.1111/j.1469-7793.2001.00395.x. J Physiol. 2001. PMID: 11454959 Free PMC article.
-
Multiple conductance states of single Ca2+-activated Cl- channels in rabbit pulmonary artery smooth muscle cells.J Physiol. 2003 Feb 15;547(Pt 1):181-96. doi: 10.1113/jphysiol.2002.033688. Epub 2003 Jan 10. J Physiol. 2003. PMID: 12562904 Free PMC article.
-
Stimulation of Ca2+-gated Cl- currents by the calcium-dependent K+ channel modulators NS1619 [1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one] and isopimaric acid.J Pharmacol Exp Ther. 2007 Jun;321(3):1075-84. doi: 10.1124/jpet.106.118786. Epub 2007 Mar 8. J Pharmacol Exp Ther. 2007. PMID: 17347326
-
Cl⁻ channels in smooth muscle cells.Pflugers Arch. 2014 May;466(5):861-72. doi: 10.1007/s00424-013-1357-2. Pflugers Arch. 2014. PMID: 24077695 Free PMC article. Review.
-
Chloride channels and their functional roles in smooth muscle tone in the vasculature.Jpn J Pharmacol. 2001 Apr;85(4):351-7. doi: 10.1254/jjp.85.351. Jpn J Pharmacol. 2001. PMID: 11388637 Review.
Cited by
-
Hypoxic pulmonary vasoconstriction.Physiol Rev. 2012 Jan;92(1):367-520. doi: 10.1152/physrev.00041.2010. Physiol Rev. 2012. PMID: 22298659 Free PMC article. Review.
-
Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels.J Gen Physiol. 2013 Oct;142(4):381-404. doi: 10.1085/jgp.201311015. J Gen Physiol. 2013. PMID: 24081981 Free PMC article.
-
Expression, localization, and functional properties of Bestrophin 3 channel isolated from mouse heart.Am J Physiol Cell Physiol. 2008 Dec;295(6):C1610-24. doi: 10.1152/ajpcell.00461.2008. Epub 2008 Oct 22. Am J Physiol Cell Physiol. 2008. PMID: 18945938 Free PMC article.
-
A minimal isoform of the TMEM16A protein associated with chloride channel activity.Biochim Biophys Acta. 2011 Sep;1808(9):2214-23. doi: 10.1016/j.bbamem.2011.05.017. Epub 2011 May 30. Biochim Biophys Acta. 2011. PMID: 21645494 Free PMC article.
-
Molecular and functional significance of Ca(2+)-activated Cl(-) channels in pulmonary arterial smooth muscle.Pulm Circ. 2015 Jun;5(2):244-68. doi: 10.1086/680189. Pulm Circ. 2015. PMID: 26064450 Free PMC article. Review.
References
-
- Boese, S.H., O. Aziz, N.L. Simmons, and M.A. Gray. 2004. Kinetics and regulation of a Ca2+-activated Cl− conductance in mouse renal inner medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 286:F682–F692. - PubMed
-
- Boton, R., D. Singer, and N. Dascal. 1990. Inactivation of calcium-activated chloride conductance in Xenopus oocytes−roles of calcium and protein kinase C. Pflugers Arch. 416:1–6. - PubMed
-
- Chipperfield, A.R., and A.A. Harper. 2000. Chloride in smooth muscle. Prog. Biophys. Mol. Biol. 74:175–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

