Absence of cochleotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times-daily tobramycin in cystic fibrosis patients
- PMID: 16801404
- PMCID: PMC1489781
- DOI: 10.1128/AAC.00995-05
Absence of cochleotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times-daily tobramycin in cystic fibrosis patients
Abstract
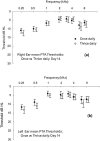
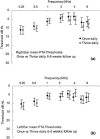
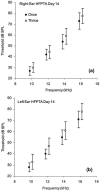
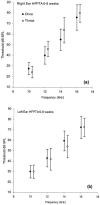
We undertook assessment of hearing in patients with cystic fibrosis who were taking part in a large randomized controlled trial of once- versus three-times-daily tobramycin for pulmonary exacerbations of cystic fibrosis (the TOPIC study). All patients were eligible to have standard pure tone audiometry performed across the frequency range of 0.25 to 8 kHz. High-frequency pure tone audiometry over 10 to 16 kHz was also performed with a subset of patients. Audiometry was undertaken at the start of tobramycin treatment, at the end of a 14-day course of treatment, and at follow-up 6 to 8 weeks later. We enrolled 244 patients, of whom 219 (125 children and 94 adults) completed treatment. Nineteen patients were excluded from analysis due to abnormal baseline audiometry. Complete pre- and posttreatment standard audiological data were obtained for 168/219 patients. We found no significant differences in hearing thresholds when they were assessed at the baseline, at the end of treatment, and at follow-up 6 to 8 weeks later were compared. In addition, no significant differences in hearing thresholds were detected between treatment regimens. Similar results were obtained for the subset of 63/168 patients who underwent high-frequency audiometry. We conclude that for a single 14-day course of tobramycin treatment in patients with cystic fibrosis with no preexisiting auditory deficit, no measurable effect on hearing was apparent with either once- or three-times-daily treatment. Estimation of the cumulative cochleotoxic risk in cystic fibrosis patients due to repeated aminoglycoside therapy, as evidenced by the patients excluded from this study due to hearing loss, also requires further characterization.
Figures
Similar articles
-
Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis--the TOPIC study: a randomised controlled trial.Lancet. 2005 Feb 12-18;365(9459):573-8. doi: 10.1016/S0140-6736(05)17906-9. Lancet. 2005. PMID: 15708100 Clinical Trial.
-
No hearing loss after repeated courses of tobramycin in cystic fibrosis patients.Acta Otolaryngol. 2010 Feb;130(2):253-8. doi: 10.3109/00016480903015150. Acta Otolaryngol. 2010. PMID: 19479457
-
Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis.Am J Respir Crit Care Med. 2003 Mar 15;167(6):841-9. doi: 10.1164/rccm.200208-855OC. Epub 2002 Dec 12. Am J Respir Crit Care Med. 2003. PMID: 12480612 Clinical Trial.
-
[Use of inhaled tobramycin in patients with cystic fibrosis].Ter Arkh. 2010;82(8):76-8. Ter Arkh. 2010. PMID: 20873251 Review. Russian.
-
Extended-interval once-daily dosing of aminoglycosides in adult and pediatric patients with cystic fibrosis.Pharmacotherapy. 2010 Jan;30(1):95-108. doi: 10.1592/phco.30.1.95. Pharmacotherapy. 2010. PMID: 20030477 Review.
Cited by
-
Evaluation of serum concentrations achieved with an empiric once-daily tobramycin dosage regimen in children and adults with cystic fibrosis.J Pediatr Pharmacol Ther. 2012 Jan;17(1):67-77. doi: 10.5863/1551-6776-17.1.67. J Pediatr Pharmacol Ther. 2012. PMID: 23118659 Free PMC article.
-
High frequency hearing thresholds and product distortion otoacoustic emissions in cystic fibrosis patients.Braz J Otorhinolaryngol. 2015 Nov-Dec;81(6):589-97. doi: 10.1016/j.bjorl.2015.08.011. Epub 2015 Sep 8. Braz J Otorhinolaryngol. 2015. PMID: 26480907 Free PMC article.
-
Chirp-Evoked Otoacoustic Emissions and Middle Ear Absorbance for Monitoring Ototoxicity in Cystic Fibrosis Patients.Ear Hear. 2018 Jan/Feb;39(1):69-84. doi: 10.1097/AUD.0000000000000464. Ear Hear. 2018. PMID: 28708814 Free PMC article.
-
Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis.Cochrane Database Syst Rev. 2019 Sep 4;9(9):CD002009. doi: 10.1002/14651858.CD002009.pub7. Cochrane Database Syst Rev. 2019. PMID: 31483853 Free PMC article.
-
Minimizing the toxicity of aminoglycosides in cystic fibrosis.J R Soc Med. 2010 Jul;103 Suppl 1(Suppl 1):S3-5. doi: 10.1258/jrsm.2010.s11002. J R Soc Med. 2010. PMID: 20573667 Free PMC article. No abstract available.
References
-
- Anonymous. 1981. Recommended procedures for pure tone audiometry using a manually operated instrument. Br. J. Audiol. 15:213-216. - PubMed
-
- Anonymous. 1985. Recommended procedures for pure tone bone conduction audiometry without masking using a manually operated instrument. Br. J. Audiol. 19:281-282. - PubMed
-
- Anonymous. 1986. Recommendations for masking in pure tone threshold audiometry. Br. J. Audiol. 20:307-314. - PubMed
-
- Anonymous. 1992. Recommended procedure for tympanometry. Br. J. Audiol. 26:255-257. - PubMed
-
- Aran, J. M. 1995. Current perspectives on inner ear toxicity. Otolaryngol.-Head Neck Surg. 112:133-144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

