Use of probabilistic modeling within a physiologically based pharmacokinetic model to predict sulfamethazine residue withdrawal times in edible tissues in swine
- PMID: 16801411
- PMCID: PMC1489760
- DOI: 10.1128/AAC.01355-05
Use of probabilistic modeling within a physiologically based pharmacokinetic model to predict sulfamethazine residue withdrawal times in edible tissues in swine
Abstract
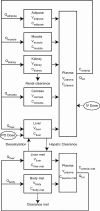
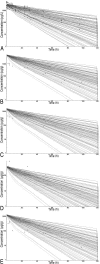
The presence of antimicrobial agents in edible tissues of food-producing animals remains a major public health concern. Probabilistic modeling techniques incorporated into a physiologically based pharmacokinetic (PBPK) model were used to predict the amounts of sulfamethazine residues in edible tissues in swine. A PBPK model for sulfamethazine in swine was adapted to include an oral dosing route. The distributions for sensitive parameters were determined and were used in a Monte Carlo analysis to predict tissue residue times. Validation of the distributions was done by comparison of the results of a Monte Carlo analysis to those obtained with an external data set from the literature and an in vivo pilot study. The model was used to predict the upper limit of the 95% confidence interval of the 99th percentile of the population, as recommended by the U.S. Food and Drug Administration (FDA). The external data set was used to calculate the withdrawal time by using the tolerance limit algorithm designed by FDA. The withdrawal times obtained by both methods were compared to the labeled withdrawal time for the same dose. The Monte Carlo method predicted a withdrawal time of 21 days, based on the amounts of residues in the kidneys. The tolerance limit method applied to the time-limited data set predicted a withdrawal time of 12 days. The existing FDA label withdrawal time is 15 days. PBPK models can incorporate probabilistic modeling techniques that make them useful for prediction of tissue residue times. These models can be used to calculate the parameters required by FDA and explore those conditions where the established withdrawal time may not be sufficient.
Figures



Similar articles
-
Tissue concentrations of sulfamethazine and tetracycline hydrochloride of swine (Sus scrofa domestica) as it relates to withdrawal methods for international export.Regul Toxicol Pharmacol. 2015 Apr;71(3):590-6. doi: 10.1016/j.yrtph.2015.02.013. Epub 2015 Feb 20. Regul Toxicol Pharmacol. 2015. PMID: 25707857
-
Use of a Monte Carlo analysis within a physiologically based pharmacokinetic model to predict doxycycline residue withdrawal time in edible tissues in swine.Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(1):73-84. doi: 10.1080/19440049.2011.624126. Epub 2011 Nov 7. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012. PMID: 22059524
-
Estimation of residue depletion of cyadox and its marker residue in edible tissues of pigs using physiologically based pharmacokinetic modelling.Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32(12):2002-17. doi: 10.1080/19440049.2015.1100330. Epub 2015 Nov 5. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015. PMID: 26414219
-
Primer on estimating withdrawal times after extralabel drug use.J Am Vet Med Assoc. 1998 Oct 1;213(7):966-8. J Am Vet Med Assoc. 1998. PMID: 9776991 Review. No abstract available.
-
Residue avoidance after topical application of veterinary drugs and parasiticides.J Am Vet Med Assoc. 1997 May 1;210(9):1288-9. J Am Vet Med Assoc. 1997. PMID: 9143531 Review.
Cited by
-
Physiological parameter values for physiologically based pharmacokinetic models in food-producing animals. Part I: Cattle and swine.J Vet Pharmacol Ther. 2020 Sep;43(5):385-420. doi: 10.1111/jvp.12861. Epub 2020 Apr 8. J Vet Pharmacol Ther. 2020. PMID: 32270548 Free PMC article. Review.
-
Physiologically-based pharmacokinetic model for evaluating gender-specific exposures of N-nitrosodimethylamine (NDMA).Arch Toxicol. 2024 Mar;98(3):821-835. doi: 10.1007/s00204-023-03652-8. Epub 2023 Dec 21. Arch Toxicol. 2024. PMID: 38127128
-
In vivo therapeutic efficacy and pharmacokinetics of colistin sulfate in an experimental model of enterotoxigenic Escherichia coli infection in weaned pigs.Vet Res. 2016 May 27;47(1):58. doi: 10.1186/s13567-016-0344-y. Vet Res. 2016. PMID: 27234971 Free PMC article.
-
Development and application of a physiologically-based pharmacokinetic model for ractopamine in goats.Front Vet Sci. 2024 Oct 2;11:1399043. doi: 10.3389/fvets.2024.1399043. eCollection 2024. Front Vet Sci. 2024. PMID: 39415957 Free PMC article.
-
Prevalence of antimicrobial residues in pork meat in Madagascar.Trop Anim Health Prod. 2014 Jan;46(1):49-55. doi: 10.1007/s11250-013-0445-9. Epub 2013 Jul 13. Trop Anim Health Prod. 2014. PMID: 23852280
References
-
- Anonymous. June 21, 2005. U.S. Department of Agriculture, Food and Drug Administration (FDA). Guidance for industry no. 3: general principles for evaluation the safety of compounds used in food producing animals. [Online.] http://www.fda.gov/cvm. Accessed September 2005.
-
- Anonymous. 2004. U.S. Department of Agriculture, Food Safety Inspection Service. Section 3, p. 13-18. [Online.] 2003 FSIS national residue program data. www.fsis.usda.gov/PDF/2003_Red_Book_Intro.pdf. Accessed February 2006.
-
- Anonymous. June 2005. U.S. Environmental Protection Agency, National Center for Environmental Assessment. Approaches for the application of physiologically based pharmacokinetic (PBPK) models and supporting data in risk assessment. EPA/600/R-05/043A external review draft. [Online.] http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=135427. Accessed September 2005.
-
- Bailer, A. J. 1997. An introduction to the use of physiologically based pharmacokinetic models in risk assessment. Stat. Methods Med. Res. 6:341-358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

