Absorption, metabolism, and excretion of [14C]viramidine in humans
- PMID: 16801414
- PMCID: PMC1489807
- DOI: 10.1128/AAC.00118-06
Absorption, metabolism, and excretion of [14C]viramidine in humans
Abstract
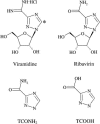
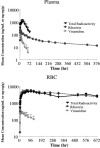
Absorption, metabolism, and excretion of [14C]viramidine, a prodrug of ribavirin, were studied in humans following a single oral dose (600 mg). Viramidine was rapidly absorbed, with a time to maximum concentration of the drug in plasma of 1.5 h. Viramidine and ribavirin accounted for only 4.3% and 42% of plasma area under the concentration-time curve (AUC) for radioactivity, respectively, indicating extensive conversion of viramidine to ribavirin, followed by further metabolism of ribavirin. The drug was largely trapped in red blood cells (RBC), with an RBC-to-plasma radioactivity AUC0-infinity ratio of 108. Excretion of total radioactivity in urine and feces accounted for 50.8% and 26.1% of the dose, respectively. The metabolic profile in urine (0 to 24 h) indicated that viramidine was excreted primarily as triazole carboxamide (TCONH2), triazole carboxylic acid nucleoside (TCOOH), and ribavirin with a small amount of unchanged viramidine, which each accounted for 64.1%, 17.0%, 15.7%, and 3.2% of urinary radioactivity, respectively. The amounts of unchanged viramidine (3.4% of dose) and ribavirin (10% of dose) in urine were small after oral administration of viramidine.
Figures
References
-
- Aora, S., C. Xu, A. Teng, J. Peterson, L. T. Yeh, R. Gish, D. Lau, S. Rossi, and C. C. Lin. 2005. Ascending multiple-dose pharmacokinetics of viramidine, a prodrug of ribavirin, in adult subjects with compensated hepatitis C infection. J. Clin. Pharmacol. 45:275-285. - PubMed
-
- Benhamou, Y., P. Pockros, M. Rodriguez-Torres, S. Gordon, M. Shiffman, Y. Lurie, N. Afdhal, K. Lamon, Y. Kim, and B. Murphy. 2006. The safety and efficacy of Viramidine® plus pegylated interferon alfa-2b versus ribavirin plus pegylated interferon alfa-2b in therapy-naïve patients infected with HCV: phase 3 results, abstr. 741. Abstr. 41st Eur. Assoc. Study of Liver. Vienna, Austria, European Association for the Study of the Liver, Geneva, Switzerland.
-
- Catlin, D. H., R. A. Smith, and A. I. Samuels. 1980. 14C-ribavirin: distribution and pharmacokinetic studies in rats, baboons and man, p. 83-98. In R. A. Smith and R. A. Kirkpatrick (ed.), Ribavirin: a broad spectrum antiviral agent. Academic Press Inc., New York, N.Y.
-
- Dadgostari, S., C. Xu, L. Yeh, C. C. Lin, and D. Vitarella. 2004. Viramidine demonstrates better safety than ribavirin in monkeys but not rats. Drug Chem. Toxicol. 27:191-211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

