In vivo fluconazole pharmacodynamics and resistance development in a previously susceptible Candida albicans population examined by microbiologic and transcriptional profiling
- PMID: 16801416
- PMCID: PMC1489797
- DOI: 10.1128/AAC.01305-05
In vivo fluconazole pharmacodynamics and resistance development in a previously susceptible Candida albicans population examined by microbiologic and transcriptional profiling
Abstract
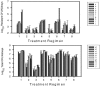
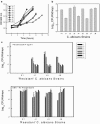
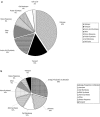
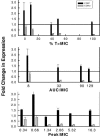
Antimicrobial drug resistance can limit the ability to effectively treat patients. Numerous factors have been proposed to impact the development of antimicrobial resistance, including those specific to the drug and the dosing regimen. The field of investigation that examines the relationship between dosing regimen and outcome is termed antimicrobial pharmacokinetics and pharmacodynamics. Our prior in vivo investigations examined the relationship between fluconazole pharmacodynamics and the modulation of isogenic resistant and susceptible Candida albicans populations in a mixed-inoculum design (1). The goal of the current studies was to examine the impact of fluconazole pharmacodynamics on resistance emergence from a susceptible parent population over time using a murine systemic-candidiasis model. Both microbiologic and transcriptional endpoints were examined during the evolution of cell populations. As in our previous investigation, the more frequently administered dosing regimen prevented the emergence of a resistant cell phenotype. Conversely, dosing regimens that produced prolonged sub-MIC concentrations were associated with resistance development. The studies also demonstrated a striking relationship between fluconazole pharmacodynamic exposures and the mRNA abundance of drug resistance-associated efflux pumps. Global transcriptional profiling of cell populations during the progressive emergence of a resistance phenotype provides insight into the mechanisms underlying this complex physiologic process.
Figures



References
-
- Andes, D., A. Lepak, A. Pitula, K. Marchillo, and J. Clark. 2005. A simple approach for estimating gene expression of Candida albicans directly from a systemic infection site. J. Infect. Dis. 192:893-900. - PubMed
-
- Baddley, J. W., and S. A. Moser. 2004. Emerging fungal resistance. Clin. Lab. Med. 24:721-735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

