Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity
- PMID: 16801424
- PMCID: PMC1489792
- DOI: 10.1128/AAC.01430-05
Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity
Abstract
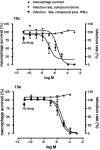
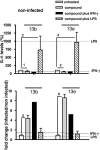
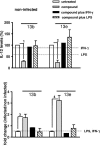
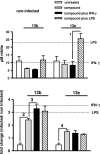
Chemotherapy of leishmaniasis is mainly based on antimonials. However, they are extremely toxic and cause serious side effects, and there is a worldwide increasing frequency of chemoresistance to antimonials. These issues emphasize the urgent need for affordable alternative drugs against leishmaniasis. Leishmania cysteine proteases are essential for parasite growth, differentiation, pathogenicity, and virulence and are thus attractive targets for combating leishmaniasis. Herein we demonstrate that the cysteine protease inhibitors aziridine-2,3-dicarboxylates 13b and 13e impaired promastigote growth at mid-micromolar concentrations and decreased the infection rate of peritoneal macrophages at concentrations 8- to 13-fold lower than those needed to inhibit parasite replication. Simultaneous treatment of infected cells with compound 13b and gamma interferon resulted in an even further reduction of the concentration needed for a significant decrease in macrophage infection rate. Notably, treatment with the compounds alone modulated the cytokine secretion of infected macrophages, with increased levels of interleukin-12 and tumor necrosis factor alpha. Furthermore, the decreased infection rate in the presence of compound 13b correlated with increased nitric oxide production by macrophages. Importantly, at the concentrations used herein, compounds 13b and 13e were not toxic against fibroblasts, macrophages, or dendritic cells. Together, these results suggest that the aziridine-2,3-dicarboxylates 13b and 13e are potential antileishmanial lead compounds with low toxicity against host cells and selective antiparasitic effects.
Figures


References
-
- Ahmed, S. A., R. M. Gogal, and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. - PubMed
-
- Alves, C. R., T. C. Benevolo-De-Andrade, J. L. Alves, and C. Pirmez. 2004. Th1 and Th2 immunological profile induced by cysteine proteinase in murine leishmaniasis. Parasite Immunol. 26:127-135. - PubMed
-
- Ascenzi, P., A. Bocedi, M. Gentile, P. Visca, and L. Gradoni. 2004. Inactivation of parasite cysteine proteinases by the NO-donor 4-(phenylsulfonyl)-3-((2-(dimethylamino)ethyl)thio)-furoxan oxalate. Biochim. Biophys. Acta 1703:69-77. - PubMed
-
- Bates, P. A., C. D. Robertson, and G. H. Coombs. 1994. Expression of cysteine proteinases by metacyclic promastigotes of Leishmania mexicana. J. Eukaryot. Microbiol. 41:199-203. - PubMed
-
- Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

