Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis
- PMID: 16801426
- PMCID: PMC1489800
- DOI: 10.1128/AAC.01468-05
Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis
Abstract
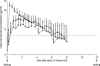
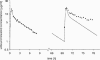
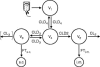
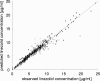
The antimicrobial agent linezolid is approved for the treatment of severe infections caused by, e.g., methicillin-resistant Staphylococcus strains. In order to evaluate the penetration of linezolid into the interstitial space fluid (ISF) of subcutaneous adipose tissue and skeletal muscle of the target population, a microdialysis study was performed with 12 patients with sepsis or septic shock after multiple intravenous infusions. Unbound linezolid concentrations were determined for plasma and microdialysates by use of a validated high-performance liquid chromatography method. Individual compartmental pharmacokinetic (PK) analysis was performed using WinNonlin. In vivo microdialysis was found to be feasible for the determination of unbound linezolid concentrations at steady state in the ISF of critically ill patients. On average, linezolid showed good distribution into ISF but with high interindividual variability. A two-compartment model was fitted to unbound concentrations in plasma with a geometric mean distribution volume of 62.9 liters and a mean clearance of 9.18 liters/h at steady state. However, disposition characteristics changed intraindividually within the time course. In addition, an integrated model for simultaneous prediction of concentrations in all matrices was developed and revealed similar results. Based on the model-predicted unbound concentrations in ISF, a scheme of more-frequent daily dosing of linezolid for some critically ill patients might be taken into consideration to avoid subinhibitory unbound concentrations in the infected tissue. The developed integrated model will be a valuable basis for further PK data analysis to explore refined dosing guidelines that achieve effective antimicrobial therapy in all patients by use of the population PK approach.
Figures



Similar articles
-
Effect of severity of sepsis on tissue concentrations of linezolid.J Antimicrob Chemother. 2008 Jan;61(1):173-6. doi: 10.1093/jac/dkm431. Epub 2007 Nov 13. J Antimicrob Chemother. 2008. PMID: 17999976 Clinical Trial.
-
Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections.Int J Antimicrob Agents. 2014 Nov;44(5):409-15. doi: 10.1016/j.ijantimicag.2014.07.001. Epub 2014 Aug 20. Int J Antimicrob Agents. 2014. PMID: 25216547
-
The effect of food on plasma and tissue concentrations of linezolid after multiple doses.Int J Antimicrob Agents. 2006 Feb;27(2):108-12. doi: 10.1016/j.ijantimicag.2005.09.017. Epub 2006 Jan 4. Int J Antimicrob Agents. 2006. PMID: 16388930
-
Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial.Clin Pharmacokinet. 2003;42(13):1129-40. doi: 10.2165/00003088-200342130-00004. Clin Pharmacokinet. 2003. PMID: 14531724 Review.
-
Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections.J Antimicrob Chemother. 2003 May;51 Suppl 2:ii17-25. doi: 10.1093/jac/dkg248. J Antimicrob Chemother. 2003. PMID: 12730139 Review.
Cited by
-
Microdialysis of Drug and Drug Metabolite: a Comprehensive In Vitro Analysis for Voriconazole and Voriconazole N-oxide.Pharm Res. 2022 Nov;39(11):2991-3003. doi: 10.1007/s11095-022-03292-0. Epub 2022 Sep 28. Pharm Res. 2022. PMID: 36171344 Free PMC article.
-
Measurement of soft tissue drug concentrations in morbidly obese and non-obese patients - A prospective, parallel group, open-labeled, controlled, phase IV, single center clinical trial.Contemp Clin Trials Commun. 2019 May 10;15:100375. doi: 10.1016/j.conctc.2019.100375. eCollection 2019 Sep. Contemp Clin Trials Commun. 2019. PMID: 31193565 Free PMC article.
-
Pharmacokinetics and penetration of linezolid into inflamed soft tissue in diabetic foot infections.Eur J Clin Pharmacol. 2008 Nov;64(11):1093-100. doi: 10.1007/s00228-008-0531-5. Epub 2008 Jul 25. Eur J Clin Pharmacol. 2008. PMID: 18654767
-
Adipocyte, adipose tissue, and infectious disease.Infect Immun. 2007 Mar;75(3):1066-78. doi: 10.1128/IAI.01455-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118983 Free PMC article. Review. No abstract available.
-
Pilot investigation on long-term subcutaneous microdialysis: proof of principle in humans.AAPS J. 2013 Jan;15(1):95-103. doi: 10.1208/s12248-012-9412-z. Epub 2012 Oct 13. AAPS J. 2013. PMID: 23065438 Free PMC article.
References
-
- Andes, D., M. L. van Ogtrop, and W. A. Craig. 1998. Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-9.
-
- Bone, R. C., W. J. Sibbald, and C. L. Sprung. 1992. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101:1481-1483. - PubMed
-
- Boselli, E., D. Breilh, T. Rimmele, S. Djabarouti, J. Toutain, D. Chassard, M. C. Saux, and B. Allaouchiche. 2005. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit. Care Med. 33:1529-1533. - PubMed
-
- Bourne, D. 1995. Mathematical modeling of pharmacokinetic data, vol. 1, p. 109. Technomic Publishing AG, Lancaster, Pa.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

