Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis
- PMID: 16801427
- PMCID: PMC1489793
- DOI: 10.1128/AAC.01520-05
Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis
Abstract
The in vitro susceptibilities of Cryptococcus neoformans isolates from consecutive human immunodeficiency virus-positive and -negative patients to the antifungal agents fluconazole, amphotericin B, and flucytosine were determined by different techniques, including the CLSI method, Etest, and broth microdilution in yeast nitrogen base (YNB) medium, during a multicenter prospective study in France. The relationship between the in vitro data and the clinical outcome 2 weeks after the initiation of antifungal therapy was assessed. In addition, the correlation between the strain serotype and the in vitro activities of the antifungals was determined, and the susceptibility results obtained with the different techniques were also compared. Thirty-seven patients received a combination of amphotericin B with flucytosine as first-line therapy, 22 were treated with amphotericin B alone, and 15 received fluconazole alone. Whatever the antifungal tested, there was no trend toward higher MICs for strains isolated from patients who failed to respond to a given therapy compared to those from patients who did not with either the CLSI method, Etest, or broth microdilution in YNB medium. The MICs obtained by the CLSI or Etest method were significantly lower for serotype D strains than for serotype A strains for both fluconazole and amphotericin B, while flucytosine MICs were not different according to serotype. These findings suggest that the in vitro antifungal susceptibility of C. neoformans, as determined with the techniques used, is not able to predict the early clinical outcome in patients with cryptococcosis.
Figures


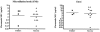
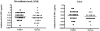
Similar articles
-
Comparison of Etests and Vitek 2 ® to broth microdilution for the susceptibility testing of Cryptococcus neoformans.Diagn Microbiol Infect Dis. 2014 Dec;80(4):294-8. doi: 10.1016/j.diagmicrobio.2014.09.006. Epub 2014 Sep 16. Diagn Microbiol Infect Dis. 2014. PMID: 25277745
-
Flucytosine and cryptococcosis: which in vitro test is the best predictor of outcome?J Chemother. 2003 Apr;15(2):124-8. doi: 10.1179/joc.2003.15.2.124. J Chemother. 2003. PMID: 12797387 Clinical Trial.
-
Comparison of the Etest and microdilution method for antifungal susceptibility testing of Cryptococcus neoformans to four antifungal agents.J Antimicrob Chemother. 2000 Dec;46(6):997-1000. doi: 10.1093/jac/46.6.997. J Antimicrob Chemother. 2000. PMID: 11102421
-
Current approach to the acute management of cryptococcal infections.J Infect. 2000 Jul;41(1):18-22. doi: 10.1053/jinf.2000.0696. J Infect. 2000. PMID: 11041709 Review. No abstract available.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
Cited by
-
Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis.Antimicrob Agents Chemother. 2008 Feb;52(2):735-8. doi: 10.1128/AAC.00990-07. Epub 2007 Dec 10. Antimicrob Agents Chemother. 2008. PMID: 18070977 Free PMC article.
-
Cryptococcus neoformans: the model organism for yeast antifungal drug susceptibility testing.Mycopathologia. 2012 Jun;173(5-6):435-43. doi: 10.1007/s11046-012-9524-0. Epub 2012 Feb 10. Mycopathologia. 2012. PMID: 22322619
-
Physiological Differences in Cryptococcus neoformans Strains In Vitro versus In Vivo and Their Effects on Antifungal Susceptibility.Antimicrob Agents Chemother. 2017 Feb 23;61(3):e02108-16. doi: 10.1128/AAC.02108-16. Print 2017 Mar. Antimicrob Agents Chemother. 2017. PMID: 28031206 Free PMC article. Review.
-
Combination of amphotericin B with flucytosine is active in vitro against flucytosine-resistant isolates of Cryptococcus neoformans.Antimicrob Agents Chemother. 2007 Jan;51(1):383-5. doi: 10.1128/AAC.00446-06. Epub 2006 Oct 16. Antimicrob Agents Chemother. 2007. PMID: 17043122 Free PMC article.
-
Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect.J Antimicrob Chemother. 2010 May;65(5):931-8. doi: 10.1093/jac/dkq046. Epub 2010 Feb 18. J Antimicrob Chemother. 2010. PMID: 20167587 Free PMC article.
References
-
- Calvo, B. M., A. L. Colombo, O. Fischman, A. Santiago, L. Thompson, M. Lazera, F. Telles, K. Fukushima, K. Nishimura, R. Tanaka, M. Myiajy, and M. L. Moretti-Branchini. 2001. Antifungal susceptibilities, varieties, and electrophoretic karyotypes of clinical isolates of Cryptococcus neoformans from Brazil, Chile, and Venezuela. J. Clin. Microbiol. 39:2348-2350. - PMC - PubMed
-
- Chen, Y. C., S. C. Chang, C. C. Shih, C. C. Hung, K. T. Luhbd, Y. S. Pan, and W. C. Hsieh. 2000. Clinical features and in vitro susceptibilities of two varieties of Cryptococcus neoformans in Taiwan. Diagn. Microbiol. Infect. Dis. 36:175-183. - PubMed
-
- Clinical and Laboratory Standards Institute. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M-27A2. Clinical and Laboratory Standards Institute, Wayne, Pa.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

