Activities of alkoxyalkyl esters of cidofovir (CDV), cyclic CDV, and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine against orthopoxviruses in cell monolayers and in organotypic cultures
- PMID: 16801436
- PMCID: PMC1489770
- DOI: 10.1128/AAC.01489-05
Activities of alkoxyalkyl esters of cidofovir (CDV), cyclic CDV, and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine against orthopoxviruses in cell monolayers and in organotypic cultures
Abstract
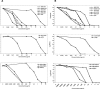
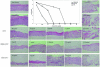
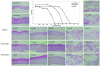
The potencies of several alkoxyalkyl esters of acyclic nucleoside phosphonates against vaccinia virus and cowpox virus were evaluated in cell monolayers and three-dimensional epithelial raft cultures. Prodrugs were at least 20-fold more active than their parent compounds. Octadecycloxyethyl-(S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine emerged as the most potent derivative.
Figures
References
-
- Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. - PMC - PubMed
-
- Berche, P. 2001. The threat of smallpox and bioterrorism. Trends Microbiol. 9:15-18. - PubMed
-
- Breman, J. G., and I. Arita. 1980. The confirmation and maintenance of smallpox eradication. N. Engl. J. Med. 303:1263-1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

