CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells
- PMID: 16801532
- PMCID: PMC1502460
- DOI: 10.1073/pnas.0604236103
CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells
Abstract
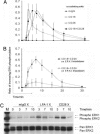
Activation of natural killer (NK) cell cytotoxicity requires adhesion and formation of a conjugate with a susceptible target cell, followed by actin polymerization, and polarization of the microtubule organizing center (MTOC) and cytolytic granules to the NK cell immune synapse. Here, by using the YTS NK cell line as a model, CD28 is shown to be an activating receptor. It signals cytotoxicity in a process dependent on phosphoinositide-3 kinase activation, leading to sustained extracellular signal-regulated kinase 2 (ERK2) phosphorylation. ERK and phospho-ERK localize to microtubule filaments. Neither conjugation with targets nor actin polymerization is affected by blocking ERK2 activation. However, both polarization of the MTOC and cytolytic granules to the synaptic region and NK cell cytotoxicity are strongly reduced by blocking ERK2 activation. A role for the CD28/CD80 interaction in cytotoxicity of human peripheral NK cells also was established. By contrast, lymphocyte function-associated antigen 1 (LFA-1) ligation transduces only a transient ERK2 activation and fails to induce killing in YTS cells. Thus, in YTS cells, a CD28 signal is used to polarize the MTOC and cytolytic granules to the NK cell immune synapse by stimulating sustained ERK2 activation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures





Similar articles
-
Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity.Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6329-34. doi: 10.1073/pnas.0611655104. Epub 2007 Mar 29. Proc Natl Acad Sci U S A. 2007. PMID: 17395718 Free PMC article.
-
Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling.Blood. 2013 Apr 4;121(14):2627-37. doi: 10.1182/blood-2012-06-437012. Epub 2013 Feb 4. Blood. 2013. PMID: 23380740 Free PMC article.
-
IQGAP1 involvement in MTOC and granule polarization in NK-cell cytotoxicity.Eur J Immunol. 2011 Sep;41(9):2763-73. doi: 10.1002/eji.201040444. Epub 2011 Aug 3. Eur J Immunol. 2011. PMID: 21681737
-
Molecular regulation of the plasma membrane-proximal cellular steps involved in NK cell cytolytic function.J Cell Sci. 2020 Feb 21;133(5):jcs240424. doi: 10.1242/jcs.240424. J Cell Sci. 2020. PMID: 32086255 Free PMC article. Review.
-
Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity.Front Immunol. 2014 Jan 21;5:2. doi: 10.3389/fimmu.2014.00002. eCollection 2014. Front Immunol. 2014. PMID: 24478771 Free PMC article. Review.
Cited by
-
Granzyme B- and Fas ligand-mediated cytotoxic function induced by mitogenic CD28 stimulation of human memory CD4+ T cells.J Leukoc Biol. 2012 May;91(5):759-71. doi: 10.1189/jlb.0511264. Epub 2012 Mar 13. J Leukoc Biol. 2012. PMID: 22416257 Free PMC article.
-
Intercellular transfer of oncogenic H-Ras at the immunological synapse.PLoS One. 2007 Nov 21;2(11):e1204. doi: 10.1371/journal.pone.0001204. PLoS One. 2007. PMID: 18030338 Free PMC article.
-
Transcriptional signature of rapidly responding NK cells reveals S1P5 and CXCR4 as anti-tumor response disruptors.Sci Rep. 2025 Mar 28;15(1):10769. doi: 10.1038/s41598-025-95211-7. Sci Rep. 2025. PMID: 40155684 Free PMC article.
-
Navigating barriers: the challenge of directed secretion at the natural killer cell lytic immunological synapse.J Clin Immunol. 2010 May;30(3):358-63. doi: 10.1007/s10875-010-9372-y. Epub 2010 Feb 27. J Clin Immunol. 2010. PMID: 20191315 Free PMC article. Review.
-
Targeting CD5 chimeric antigen receptor-engineered natural killer cells against T-cell malignancies.Exp Hematol Oncol. 2024 Oct 26;13(1):104. doi: 10.1186/s40164-024-00577-5. Exp Hematol Oncol. 2024. PMID: 39462383 Free PMC article.
References
-
- Janeway C. A., Jr, Medzhitov R. Annu. Rev. Immunol. 2002;20:197–216. - PubMed
-
- Chiesa S., Tomasello T., Vivier E., Vely F. Mol. Immunol. 2005;42:477–484. - PubMed
-
- Luque I., Reyburn H., Strominger J. L. Hum. Immunol. 2000;61:721–728. - PubMed
-
- Galea-Lauri J., Darling D., Gan S. U., Krivochtchapov L., Kuiper M., Gaken J., Souberbielle B., Farzaneh F. J. Immunol. 1999;163:62–70. - PubMed
-
- Wilson J. L., Charo J., Martin-Fontecha A., Dellabona P., Casorati G., Chambers B. J., Kiessling R., Bejarano M. T., Ljunggren H. G. J. Immunol. 1999;163:4207–4212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

