Effective gene therapy in an authentic model of Tay-Sachs-related diseases
- PMID: 16801539
- PMCID: PMC1482797
- DOI: 10.1073/pnas.0603765103
Effective gene therapy in an authentic model of Tay-Sachs-related diseases
Abstract
Tay-Sachs disease is a prototypic neurodegenerative disease. Lysosomal storage of GM2 ganglioside in Tay-Sachs and the related disorder, Sandhoff disease, is caused by deficiency of beta-hexosaminidase A, a heterodimeric protein. Tay-Sachs-related diseases (GM2 gangliosidoses) are incurable, but gene therapy has the potential for widespread correction of the underlying lysosomal defect by means of the secretion-recapture cellular pathway for enzymatic complementation. Sandhoff mice, lacking the beta-subunit of hexosaminidase, manifest many signs of classical human Tay-Sachs disease and, with an acute course, die before 20 weeks of age. We treated Sandhoff mice by stereotaxic intracranial inoculation of recombinant adeno-associated viral vectors encoding the complementing human beta-hexosaminidase alpha and beta subunit genes and elements, including an HIV tat sequence, to enhance protein expression and distribution. Animals survived for >1 year with sustained, widespread, and abundant enzyme delivery in the nervous system. Onset of the disease was delayed with preservation of motor function; inflammation and GM2 ganglioside storage in the brain and spinal cord was reduced. Gene delivery of beta-hexosaminidase A by using adeno-associated viral vectors has realistic potential for treating the human Tay-Sachs-related diseases.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
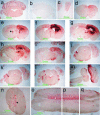
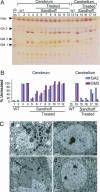

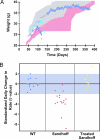
Similar articles
-
Pronounced Therapeutic Benefit of a Single Bidirectional AAV Vector Administered Systemically in Sandhoff Mice.Mol Ther. 2020 Oct 7;28(10):2150-2160. doi: 10.1016/j.ymthe.2020.06.021. Epub 2020 Jun 19. Mol Ther. 2020. PMID: 32592687 Free PMC article.
-
Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases.Hum Mol Genet. 1997 Oct;6(11):1879-85. doi: 10.1093/hmg/6.11.1879. Hum Mol Genet. 1997. PMID: 9302266
-
In cellulo examination of a beta-alpha hybrid construct of beta-hexosaminidase A subunits, reported to interact with the GM2 activator protein and hydrolyze GM2 ganglioside.PLoS One. 2013;8(3):e57908. doi: 10.1371/journal.pone.0057908. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23483939 Free PMC article.
-
[Molecular pathogenesis and therapeutic approach of GM2 gangliosidosis].Yakugaku Zasshi. 2013;133(2):269-74. doi: 10.1248/yakushi.12-00199. Yakugaku Zasshi. 2013. PMID: 23370522 Review. Japanese.
-
Biology and potential strategies for the treatment of GM2 gangliosidoses.Mol Med Today. 1998 Apr;4(4):158-65. doi: 10.1016/s1357-4310(98)01227-1. Mol Med Today. 1998. PMID: 9572057 Review.
Cited by
-
A novel gene editing system to treat both Tay-Sachs and Sandhoff diseases.Gene Ther. 2020 May;27(5):226-236. doi: 10.1038/s41434-019-0120-5. Epub 2020 Jan 2. Gene Ther. 2020. PMID: 31896760 Free PMC article.
-
Natural history of Tay-Sachs disease in sheep.Mol Genet Metab. 2021 Sep-Oct;134(1-2):164-174. doi: 10.1016/j.ymgme.2021.08.009. Epub 2021 Aug 21. Mol Genet Metab. 2021. PMID: 34456134 Free PMC article.
-
Construction of a hybrid β-hexosaminidase subunit capable of forming stable homodimers that hydrolyze GM2 ganglioside in vivo.Mol Ther Methods Clin Dev. 2016 Mar 2;3:15057. doi: 10.1038/mtm.2015.57. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 26966698 Free PMC article.
-
Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy.Mol Ther. 2013 Jul;21(7):1306-15. doi: 10.1038/mt.2013.86. Epub 2013 May 21. Mol Ther. 2013. PMID: 23689599 Free PMC article.
-
Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications.J Funct Biomater. 2021 May 11;12(2):32. doi: 10.3390/jfb12020032. J Funct Biomater. 2021. PMID: 34064736 Free PMC article.
References
-
- Gravel R. A., Kaback M. M., Proia R. L., Sandhoff K., Suzuki K., Suzuki K. In: The Metabolic and Molecular Bases of Inherited Disease. 8th Ed. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., editors. Vol. 3. New York: McGraw–Hill; 2001. pp. 3827–3876.
-
- Cork L. C., Munnell J. F., Lorenz M. D., Murphy J. V., Baker H. J., Rattazzi M. C. Science. 1977;196:1014–1017. - PubMed
-
- Fox J., Li Y. T., Dawson G. Acta Neuropathol. 1999;97:57–62. - PubMed
-
- Sango K., Yamanaka S., Hoffmann A., Okuda Y., Grinberg A., Westphal H., McDonald M. P., Crawley J. N., Sandhoff K., Suzuki K., Proia R. L. Nat. Genet. 1995;11:170–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

