Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome
- PMID: 16801550
- PMCID: PMC1502447
- DOI: 10.1073/pnas.0601058103
Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome
Abstract
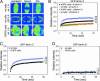
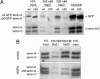
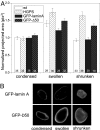
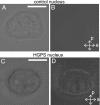
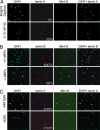
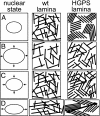
The nuclear lamina is a network of structural filaments, the A and B type lamins, located at the nuclear envelope and throughout the nucleus. Lamin filaments provide the nucleus with mechanical stability and support many basic activities, including gene regulation. Mutations in LMNA, the gene encoding A type lamins, cause numerous human diseases, including the segmental premature aging disease Hutchinson-Gilford progeria syndrome (HGPS). Here we show that structural and mechanical properties of the lamina are altered in HGPS cells. We demonstrate by live-cell imaging and biochemical analysis that lamins A and C become trapped at the nuclear periphery in HGPS patient cells. Using micropipette aspiration, we show that the lamina in HGPS cells has a significantly reduced ability to rearrange under mechanical stress. Based on polarization microscopy results, we suggest that the lamins are disordered in the healthy nuclei, whereas the lamins in HGPS nuclei form orientationally ordered microdomains. The reduced deformability of the HGPS nuclear lamina possibly could be due to the inability of these orientationally ordered microdomains to dissipate mechanical stress. Surprisingly, intact HGPS cells exhibited a degree of resistance to acute mechanical stress similar to that of cells from healthy individuals. Thus, in contrast to the nuclear fragility seen in lmna null cells, the lamina network in HGPS cells has unique mechanical properties that might contribute to disease phenotypes by affecting responses to mechanical force and misregulation of mechanosensitive gene expression.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- DeBusk F. L. J. Pediatr. 1972;80:697–724. - PubMed
-
- Uitto J. Trends. Mol. Med. 2002;8:155–157. - PubMed
-
- De Sandre-Giovannoli A., Bernard R., Cau P., Navarro C., Amiel J., Boccaccio I., Lyonnet S., Stewart C. L., Munnich A., Le Merrer M., Levy N. Science. 2003;300:2055. - PubMed
-
- Hutchison C. J. Nat. Rev. Mol. Cell Biol. 2002;3:848–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

