Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils
- PMID: 16802344
- PMCID: PMC3119431
- DOI: 10.1002/art.21930
Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils
Abstract
Objective: Synovial fibroblasts share a number of phenotype markers with fibroblasts derived from bone marrow. In this study we investigated the role of matched fibroblasts obtained from 3 different sources (bone marrow, synovium, and skin) to test the hypothesis that synovial fibroblasts share similarities with bone marrow-derived fibroblasts in terms of their ability to support survival of T cells and neutrophils.
Methods: Matched synovial, bone marrow, and skin fibroblasts were established from 8 different patients with rheumatoid arthritis who were undergoing knee or hip surgery. Resting or activated fibroblasts were cocultured with either CD4 T cells or neutrophils, and the degree of leukocyte survival, apoptosis, and proliferation were measured.
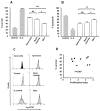
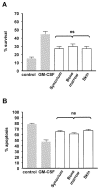
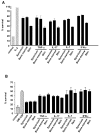
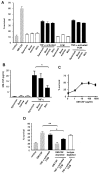
Results: Fibroblasts derived from all 3 sites supported increased survival of CD4 T cells, mediated principally by interferon-beta. However, synovial and bone marrow fibroblasts shared an enhanced site-specific ability to maintain CD4 T cell survival in the absence of proliferation, an effect that was independent of fibroblast activation or proliferation but required direct T cell-fibroblast cell contact. In contrast, fibroblast-mediated neutrophil survival was less efficient, being independent of the site of origin of the fibroblast but dependent on prior fibroblast activation, and mediated solely by soluble factors, principally granulocyte-macrophage colony-stimulating factor.
Conclusion: These results suggest an important functional role for fibroblasts in the differential accumulation of leukocyte subsets in a variety of tissue microenvironments. The findings also provide a potential explanation for site-specific differences in the pattern of T cell and neutrophil accumulation observed in chronic inflammatory diseases.
Figures


References
-
- Postlethwaite AE. Role of T cells and cytokines in effecting fibrosis. Int Rev Immunol. 1995;12:247–58. - PubMed
-
- Rezzonico R, Burger D, Dayer JM. Direct contact between T lymphocytes and human dermal fibroblasts or synoviocytes down-regulates types I and III collagen production via cell-associated cytokines. J Biol Chem. 1998;273:18720–8. - PubMed
-
- Yamamura Y, Gupta R, Morita Y, He X, Pai R, Endres J, et al. Effector function of resting T cells: activation of synovial fibroblasts. J Immunol. 2001;166:2270–5. - PubMed
-
- Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez DA, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

