Effects of human gamma-globin in murine beta-thalassaemia
- PMID: 16803575
- PMCID: PMC2811697
- DOI: 10.1111/j.1365-2141.2006.06102.x
Effects of human gamma-globin in murine beta-thalassaemia
Abstract
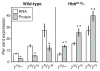
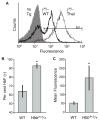
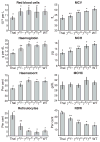
Murine models of beta-thalassaemia have been used to test therapeutic globin gene vectors. However, the level of gamma-globin expression necessary to achieve full phenotypic correction in these models is unclear. In order to address this issue, we carried out breeding and transplantation studies in murine models of beta-thalassaemia intermedia (Hbb(th-3)/+) and severe beta-thalassaemia major (Hbb(th-3)/Hbb(th-3)) using transgenic lines expressing various levels of human gamma-globin. Expression of gamma-globin RNA at a modest 7-14% of total alpha-globin RNA resulted in the selective survival of HbF(+) erythrocytes, a fivefold increase in total HbF, and a phenotypic improvement in the beta-thalassaemia intermedia model. Full normalisation of erythrocyte indices in this model required gamma-globin RNA expression at 27% of alpha-globin, resulting in an average 40% (6.8 g/dl) HbF. Studies using the homozygous Hbb(th-3) model of lethal beta-thalassaemia major demonstrated that even this high level of gamma-globin expression, for reasons related to the function of the hybrid globin tetramers, could only prolong, but not fully support, survival. Taken together, these results indicate that only the heterozygous Hbb(th-3) model of beta-thalassaemia intermedia can be reliably used for the pre-clinical assessment of gamma-globin gene therapy vectors, as well as other means of gamma-globin gene induction.
Figures
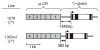


References
-
- Antonchuck J, Hyland CD, Hilton DJ, Alexander WS. Synergistic effects of erythropoiesis, thrombopoiesis, and stem cell competitiveness in mice deficient in thrombopoietin and steel factor receptors. Blood. 2004;104:1306–1313. - PubMed
-
- Charache S, Clegg JB, Weatherall DJ. The negro variety of hereditary persistence of fetal haemoglobin is a mild form of thalassaemia. British Journal of Haematology. 1976;34:527–534. - PubMed
-
- Constantoulakis P, Josephson B, Mangahas L, Papayannopoulou T, Enver T, Costantini F, Stamatoyannopoulos G. Locus control region – a gamma transgenic mice: a new model for studying the induction of fetal hemoglobin in the adult. Blood. 1991;77:1326–1333. - PubMed
-
- Davis BH, Ornvold K, Bigelow NC. Flow cytometric reticulocyte maturity index: a useful laboratory parameter of erythropoietic activity in anemia. Cytometry. 1995;22:35–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

