Irreversible aggregation of protein synthesis machinery after focal brain ischemia
- PMID: 16805800
- PMCID: PMC3518070
- DOI: 10.1111/j.1471-4159.2006.03838.x
Irreversible aggregation of protein synthesis machinery after focal brain ischemia
Abstract
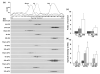
Focal brain ischemia leads to a slow type of neuronal death in the penumbra that starts several hours after ischemia and continues to mature for days. During this maturation period, blood flow, cellular ATP and ionic homeostasis are gradually recovered in the penumbral region. In striking contrast, protein synthesis is irreversibly inhibited. This study used a rat focal brain ischemia model to investigate whether or not irreversible translational inhibition is due to abnormal aggregation of translational complex components, i.e. the ribosomes and their associated nascent polypeptides, protein synthesis initiation factors and co-translational chaperones. Under electron microscopy, most rosette-shaped polyribosomes were relatively evenly distributed in the cytoplasm of sham-operated control neurons, but clumped into large abnormal aggregates in penumbral neurons subjected to 2 h of focal ischemia followed by 4 h of reperfusion. The abnormal ribosomal protein aggregation lasted until the onset of delayed neuronal death at 24-48 h of reperfusion after ischemia. Biochemical study further suggested that translational complex components, including small ribosomal subunit protein 6 (S6), large subunit protein 28 (L28), eukaryotic initiation factors 2alpha, 4E and 3eta, and co-translational chaperone heat-shock cognate protein 70 (HSC70) and co-chaperone Hdj1, were all irreversibly clumped into large abnormal protein aggregates after ischemia. Translational complex components were also highly ubiquitinated. This study clearly demonstrates that focal ischemia leads to irreversible aggregation of protein synthesis machinery that contributes to neuronal death after focal brain ischemia.
Figures



Similar articles
-
Co-translational protein aggregation after transient cerebral ischemia.Neuroscience. 2005;134(4):1273-84. doi: 10.1016/j.neuroscience.2005.05.015. Neuroscience. 2005. PMID: 16039801 Free PMC article.
-
Protein aggregation after focal brain ischemia and reperfusion.J Cereb Blood Flow Metab. 2001 Jul;21(7):865-75. doi: 10.1097/00004647-200107000-00012. J Cereb Blood Flow Metab. 2001. PMID: 11435799
-
The intraischemic and early reperfusion changes of protein synthesis in the rat brain. eIF-2 alpha kinase activity and role of initiation factors eIF-2 alpha and eIF-4E.J Cereb Blood Flow Metab. 1998 Jan;18(1):59-66. doi: 10.1097/00004647-199801000-00006. J Cereb Blood Flow Metab. 1998. PMID: 9428306
-
Irreversible translation arrest in the reperfused brain.J Cereb Blood Flow Metab. 2007 May;27(5):875-93. doi: 10.1038/sj.jcbfm.9600388. Epub 2006 Aug 16. J Cereb Blood Flow Metab. 2007. PMID: 16926841 Free PMC article. Review.
-
Molecular and biochemical events within the brain subjected to cerebral ischemia (targets for therapeutical intervention).Clin Neurosci. 1997;4(4):179-83. Clin Neurosci. 1997. PMID: 9186039 Review.
Cited by
-
Protein Aggregation in the Pathogenesis of Ischemic Stroke.Cell Mol Neurobiol. 2021 Aug;41(6):1183-1194. doi: 10.1007/s10571-020-00899-y. Epub 2020 Jun 11. Cell Mol Neurobiol. 2021. PMID: 32529541 Free PMC article. Review.
-
C-terminus of heat shock cognate 70 interacting protein increases following stroke and impairs survival against acute oxidative stress.Antioxid Redox Signal. 2011 May 15;14(10):1787-801. doi: 10.1089/ars.2010.3300. Epub 2010 Dec 2. Antioxid Redox Signal. 2011. PMID: 20677910 Free PMC article.
-
Translation arrest and ribonomics in post-ischemic brain: layers and layers of players.J Neurochem. 2008 Sep;106(6):2288-301. doi: 10.1111/j.1471-4159.2008.05561.x. Epub 2008 Jul 8. J Neurochem. 2008. PMID: 18627434 Free PMC article. Review.
-
Protein aggregation and proteasome dysfunction after brain ischemia.Stroke. 2007 Dec;38(12):3230-6. doi: 10.1161/STROKEAHA.107.487108. Epub 2007 Nov 1. Stroke. 2007. PMID: 17975104 Free PMC article.
-
The transcriptome of cerebral ischemia.Brain Res Bull. 2012 Jul 1;88(4):313-9. doi: 10.1016/j.brainresbull.2012.02.002. Epub 2012 Feb 21. Brain Res Bull. 2012. PMID: 22381515 Free PMC article. Review.
References
-
- Althausen S, Mengesdorf T, Mies G, Olah L, Nairn AC, Proud CG, Paschen W. Changes in the phosphorylation of initiation factor eIF-2alpha, elongation factor eEF-2 and p70, S6 kinase after transient focal cerebral ischaemia in mice. J. Neurochem. 2001;78:779–787. - PubMed
-
- Angelidis CE, Lazaridis I, Pagoulatos GN. Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur. J. Biochem. 1999;259:505–512. - PubMed
-
- Bukau B, Hesterkamp T, Luirink J. Growing up in a dangerous environment: a network of multiple targeting and folding pathways for nascent polypeptides in the cytosol. Trends Cell Biol. 1996;6:480–486. - PubMed
-
- DeGracia DJ. Acute and persistent protein synthesis inhibition following cerebral reperfusion. J. Neurosci. Res. 2004;77:771–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

