Plausible linkage of hypoxia inducible factor-1alpha in uterine cervical cancer
- PMID: 16805819
- PMCID: PMC11158725
- DOI: 10.1111/j.1349-7006.2006.00262.x
Plausible linkage of hypoxia inducible factor-1alpha in uterine cervical cancer
Abstract
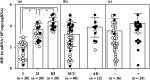
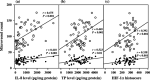
Angiogenesis is essential for the development, growth and advancement of solid tumors. Angiogenesis is induced by hypoxia with angiogenic transcription factor hypoxia inducible factors (HIF). This prompted us to study the clinical implications of HIF relative to angiogenesis in uterine cervical cancers. Although there was no significant difference in HIF-1alpha histoscores and mRNA levels according to histopathological type or lymph node metastasis, HIF-1alpha histoscores and mRNA levels increased significantly with advancing cancer stages. The prognosis of 30 patients with high HIF-1alpha in uterine cervical cancers was poor (73% survival), whereas the 24-month survival rate of the other 30 patients with low HIF-1alpha was 93%. HIF-1alpha histoscores and mRNA levels were correlated with the levels of the angiogenic factors thymidine phosphorylase and interleukin-8, and HIF-1alpha might be linked with these factors in cervical cancer tissue. HIF-1alpha is a candidate for prognostic indicator as an angiogenic mediator in uterine cervical cancer.
Figures







Similar articles
-
Plausible linkage of hypoxia-inducible factor (HIF) in uterine endometrial cancers.Oncology. 2006;71(1-2):95-101. doi: 10.1159/000100477. Epub 2007 Mar 6. Oncology. 2006. PMID: 17341889
-
Elevated expression of thymosin β4, vascular endothelial growth factor (VEGF), and hypoxia inducible factor (HIF)-1α in early-stage cervical cancers.Pathol Oncol Res. 2011 Sep;17(3):493-502. doi: 10.1007/s12253-010-9327-x. Epub 2011 Jan 7. Pathol Oncol Res. 2011. PMID: 21213129
-
Upregulation of hypoxia inducible factor 1alpha mRNA is associated with elevated vascular endothelial growth factor expression and excessive angiogenesis and predicts a poor prognosis in gastric carcinoma.World J Gastroenterol. 2007 Mar 21;13(11):1680-6. doi: 10.3748/wjg.v13.i11.1680. World J Gastroenterol. 2007. PMID: 17461470 Free PMC article.
-
Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma.Cancer. 2002 Sep 1;95(5):1055-63. doi: 10.1002/cncr.10774. Cancer. 2002. PMID: 12209691
-
How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer?Ann Diagn Pathol. 2013 Jun;17(3):305-11. doi: 10.1016/j.anndiagpath.2012.12.002. Epub 2013 Feb 1. Ann Diagn Pathol. 2013. PMID: 23375385 Review.
Cited by
-
Novel therapeutic strategy for uterine endometrial cancers.Int J Clin Oncol. 2008 Oct;13(5):411-5. doi: 10.1007/s10147-008-0825-8. Epub 2008 Oct 23. Int J Clin Oncol. 2008. PMID: 18946751 Review.
-
Overexpression of inhibitor of DNA-binding (ID)-1 protein related to angiogenesis in tumor advancement of ovarian cancers.BMC Cancer. 2009 Dec 10;9:430. doi: 10.1186/1471-2407-9-430. BMC Cancer. 2009. PMID: 20003244 Free PMC article.
References
-
- Folkman J. Tumor angiogenesis. Adv Cancer Res 1985; 43: 175–203. - PubMed
-
- Wang GL, Semenza GL. Purification and characterization of hypoxia‐inducible factor 1. J Biol Chem 1995; 270: 1230–7. - PubMed
-
- Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia‐inducible transcription factor depends primarily upon redox‐sensitive stabilization of its alpha subunit. J Biol Chem 1996; 271: 32253–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

