TRPM4 controls insulin secretion in pancreatic beta-cells
- PMID: 16806463
- PMCID: PMC5663640
- DOI: 10.1016/j.ceca.2006.04.032
TRPM4 controls insulin secretion in pancreatic beta-cells
Abstract
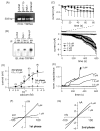
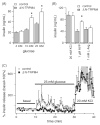
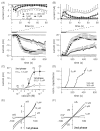
TRPM4 is a calcium-activated non-selective cation channel that is widely expressed and proposed to be involved in cell depolarization. In excitable cells, TRPM4 may regulate calcium influx by causing the depolarization that drives the activation of voltage-dependent calcium channels. We here report that insulin-secreting cells of the rat pancreatic beta-cell line INS-1 natively express TRPM4 proteins and generate large depolarizing membrane currents in response to increased intracellular calcium. These currents exhibit the characteristics of TRPM4 and can be suppressed by expressing a dominant negative TRPM4 construct, resulting in significantly decreased insulin secretion in response to a glucose stimulus. Reduced insulin secretion was also observed with arginine vasopressin stimulation, a Gq-coupled receptor agonist in beta-cells. Moreover, the recruitment of TRPM4 currents was biphasic in both INS-1 cells as well as HEK-293 cells overexpressing TRPM4. The first phase is due to activation of TRPM4 channels localized within the plasma membrane followed by a slower secondary phase, which is caused by the recruitment of TRPM4-containing vesicles to the plasma membrane during exocytosis. The secondary phase can be observed during perfusion of cells with increasing [Ca(2+)](i), replicated with agonist stimulation, and coincides with an increase in cell capacitance, loss of FM1-43 dye, and vesicle fusion. Our data suggest that TRPM4 may play a key role in the control of membrane potential and electrical activity of electrically excitable secretory cells and the dynamic translocation of TRPM4 from a vesicular pool to the plasma membrane via Ca(2+)-dependent exocytosis may represent a key short- and midterm regulatory mechanism by which cells regulate electrical activity.
Figures



References
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. - PubMed
-
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–231. - PubMed
-
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

