The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture
- PMID: 16807294
- PMCID: PMC1502299
- DOI: 10.1073/pnas.0601956103
The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture
Abstract
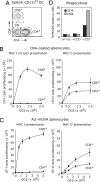
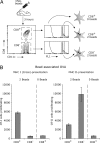
Mouse spleens contain three populations of conventional (CD11c(high)) dendritic cells (DCs) that play distinct functions. The CD8(+) DC are unique in that they can present exogenous antigens on their MHC class I molecules, a process known as cross-presentation. It is unclear whether this special ability is because only the CD8(+) DC can capture the antigens used in cross-presentation assays, or because this is the only DC population that possesses specialized machinery for cross-presentation. To solve this important question we examined the splenic DC subsets for their ability to both present via MHC class II molecules and cross-present via MHC class I using four different forms of the model antigen ovalbumin (OVA). These forms include a cell-associated form, a soluble form, OVA expressed in bacteria, or OVA bound to latex beads. With the exception of bacterial antigen, which was poorly cross-presented by all DC, all antigenic forms were cross-presented much more efficiently by the CD8(+) DC. This pattern could not be attributed simply to a difference in antigen capture because all DC subsets presented the antigen via MHC class II. Indeed, direct assessments of endocytosis showed that CD8(+) and CD8(-) DC captured comparable amounts of soluble and bead-associated antigen, yet only the CD8(+) DC cross-presented these antigenic forms. Our results indicate that cross-presentation requires specialized machinery that is expressed by CD8(+) DC but largely absent from CD8(-) DC. This conclusion has important implications for the design of vaccination strategies based on antigen targeting to DC.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Wilson N. S., Villadangos J. A. Adv. Immunol. 2005;86:241–305. - PubMed
-
- Cresswell P., Ackerman A. L., Giodini A., Peaper D. R., Wearsch P. A. Immunol. Rev. 2005;207:145–157. - PubMed
-
- Touret N., Paroutis P., Terebiznik M., Harrison R. E., Trombetta S., Pypaert M., Chow A., Jiang A., Shaw J., Yip C., et al. Cell. 2005;123:157–170. - PubMed
-
- Shortman K., Liu Y. J. Nat. Rev. Immunol. 2002;2:151–161. - PubMed
-
- Villadangos J. A., Heath W. R. Semin. Immunol. 2005;17:262–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

