Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses
- PMID: 16807324
- PMCID: PMC6673910
- DOI: 10.1523/JNEUROSCI.1382-06.2006
Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses
Abstract
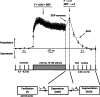
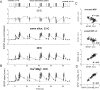
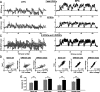
Studies of short-term plasticity (STP) in the hippocampus, performed mostly at room temperature, have shown that small central synapses rapidly depress in response to high-frequency stimulation. This decrease in synaptic strength with synapse use places constraints on the use of STP as a dynamic filter for processing of natural high-frequency input. Here we report that, because of a strong but differential temperature dependence of STP components, the properties of STP in excitatory hippocampal synapses change dramatically with temperature. By separating the contributions of various STP processes during spike trains at different temperatures, we found a shift from dominating depression at 23 degrees C to prevailing facilitation and augmentation at 33-38 degrees C. This shift of balance among STP components resulted from a large increase in amplitudes of facilitation and augmentation (Q10 approximately 2.6 and approximately 5.1, respectively) and little change in the amplitude of depression (Q10 approximately 1.1) with temperature. These changes were accompanied by the accelerated decay of all three processes (Q10 = 3.2, 6.6, and 2.1, respectively). The balance of STP components achieved at higher temperatures greatly improved the maintenance of synaptic strength during prolonged synaptic use and had a strong effect on the processing of natural spike trains: a variable mixture of facilitated and depressed responses at 23 degrees C changed into a significantly more reproducible and depression-free filtering pattern at 33-38 degrees C. This filtering pattern was highly conserved among cells, slices, and animals, and under various physiological conditions, arguing for its physiological significance. Therefore, the fine balance among STP components, achieved only at near body temperatures, is required for the robust function of STP as a dynamic filter during natural stimulation.
Figures






References
-
- Abbott LF, Regehr WG (2004). Synaptic computation. Nature 431:796–803. - PubMed
-
- Abbott LF, Varela JA, Sen K, Nelson SB (1997). Synaptic depression and cortical gain control. Science 275:220–224. - PubMed
-
- Blaustein MP, Lederer WJ (1999). Sodium/calcium exchange: its physiological implications. Physiol Rev 79:763–854. - PubMed
-
- Bolshakov VY, Siegelbaum SA (1995). Regulation of hippocampal transmitter release during development and long-term potentiation. Science 269:1730–1734. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
