Brief bursts of parallel fiber activity trigger calcium signals in bergmann glia
- PMID: 16807325
- PMCID: PMC6673913
- DOI: 10.1523/JNEUROSCI.0613-06.2006
Brief bursts of parallel fiber activity trigger calcium signals in bergmann glia
Abstract
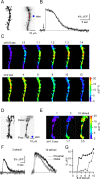
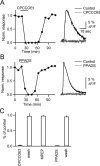
Changes in synaptic strength during ongoing activity are often mediated by neuromodulators. At the synapse between cerebellar granule cell parallel fibers (PFs) and Purkinje cells (PCs), brief bursts of stimuli can evoke endocannabinoid release from PCs and GABA release from interneurons that both inhibit transmission by activating presynaptic G-protein-coupled receptors. Studies in several brain regions suggest that synaptic activity can also evoke calcium signals in astrocytes, thereby causing them to release a transmitter, which acts presynaptically to regulate neurotransmitter release. In the cerebellum, Bergmann glia cells (BGs) are intimately associated with PF synapses. However, the mechanisms leading to calcium signals in BGs under physiological conditions and the role of BGs in regulating ongoing synaptic transmission are poorly understood. We found that brief bursts of PF activity evoke calcium signals in BGs that are triggered by the activation of metabotropic glutamate receptor 1 and purinergic receptors and mediated by calcium release from IP3-sensitive internal stores. We found no evidence for modulation of release from PFs mediated by BGs, even when endocannabinoid- and GABA-mediated presynaptic modulation was prominent. Thus, despite the fact that PF activation can reliably evoke calcium transients within BGs, it appears that BGs do not regulate synaptic transmission on the time scale of seconds to tens of seconds. Instead, endocannabinoid release from PCs and GABA release from molecular layer interneurons provide the primary means of feedback that dynamically regulate release from PF synapses.
Figures





References
-
- Auld DS, Robitaille R (2003). Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron 40:389–400. - PubMed
-
- Brasnjo G, Otis TS (2001). Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron 31:607–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
