Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2
- PMID: 16807331
- PMCID: PMC6673909
- DOI: 10.1523/JNEUROSCI.0573-06.2006
Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2
Erratum in
- J Neurosci. 2006 Jul 12;26(28):7541
Abstract
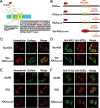
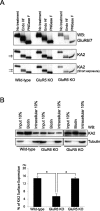
Neuronal kainate receptors are typically heteromeric complexes composed of GluR5-7 and KA1-2 subunits. Although GluR5-7 can exist as functional homomeric channels, the KA subunits cannot. KA2 is widely expressed in the CNS, and KA2/GluR6 heteromers are the most prevalent subunit composition in brain. Previous work has identified endoplasmic reticulum (ER)-retention motifs in the C terminus of KA2, which prevent surface expression of KA2 homomers. However, we find that, when these motifs are mutated, only a small fraction of KA2 is surface expressed. We now identify an additional ER retention motif in the intracellular loop region of KA2, which, when mutated together with the C-terminal motifs, significantly increases the level of KA2 surface expression. However, electrophysiological analysis of surface-expressed KA2 homomers indicates that they do not form functional ion channels. In heterologous cells, a large fraction of KA2 remains intracellular even when the trafficking motifs are mutated or when GluR6 is coexpressed. Therefore, we analyzed the trafficking of endogenous KA2 in vivo. We find that native KA2 surface expression is dramatically reduced in GluR6 knock-out mice compared with wild-type mice. In contrast, KA2 trafficking was unaffected in the GluR5 knock-out. Thus, our study demonstrates that trafficking motifs in both the intracellular loop and C terminus regulate KA2 surface expression; however, in neurons, GluR6 oligomerization is required for egress of KA2 from the ER and transport to the cell surface. The combination of these mechanisms likely prevents surface expression of nonfunctional KA2 homomers and ensures a high level of GluR6/KA2 heteromeric kainate receptors.
Figures



References
-
- Coussen F, Perrais D, Jaskolski F, Sachidhanandam S, Normand E, Bockaert J, Marin P, Mulle C (2005). Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron 47:555–566. - PubMed
-
- Ellgaard L, Helenius A (2003). Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
