The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15
- PMID: 16807332
- PMCID: PMC6673907
- DOI: 10.1523/JNEUROSCI.1163-06.2006
The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15
Abstract
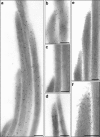
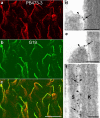
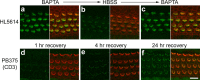
Sound and acceleration are detected by hair bundles, mechanosensory structures located at the apical pole of hair cells in the inner ear. The different elements of the hair bundle, the stereocilia and a kinocilium, are interconnected by a variety of link types. One of these links, the tip link, connects the top of a shorter stereocilium with the lateral membrane of an adjacent taller stereocilium and may gate the mechanotransducer channel of the hair cell. Mass spectrometric and Western blot analyses identify the tip-link antigen, a hitherto unidentified antigen specifically associated with the tip and kinocilial links of sensory hair bundles in the inner ear and the ciliary calyx of photoreceptors in the eye, as an avian ortholog of human protocadherin-15, a product of the gene for the deaf/blindness Usher syndrome type 1F/DFNB23 locus. Multiple protocadherin-15 transcripts are shown to be expressed in the mouse inner ear, and these define four major isoform classes, two with entirely novel, previously unidentified cytoplasmic domains. Antibodies to the three cytoplasmic domain-containing isoform classes reveal that each has a different spatiotemporal expression pattern in the developing and mature inner ear. Two isoforms are distributed in a manner compatible for association with the tip-link complex. An isoform located at the tips of stereocilia is sensitive to calcium chelation and proteolysis with subtilisin and reappears at the tips of stereocilia as transduction recovers after the removal of calcium chelators. Protocadherin-15 is therefore associated with the tip-link complex and may be an integral component of this structure and/or required for its formation.
Figures







References
-
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, Weil D, Yonekawa H, Wolfrum U, El-Amraoui A, Petit C (2005). Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet 14:347–356. - PubMed
-
- Ahmed ZM, Riazuddin S, Ahmad J, Bernstein SL, Guo Y, Sabar MF, Sieving P, Griffith AJ, Friedman TB, Belyantseva IA, Wilcox ER (2003). PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet 12:3215–3223. - PubMed
-
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP (2001a). The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27:99–102. - PubMed
-
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, Scharwtz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hageman GS, Woychik RP, Smith RJ (2001b). Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10:1709–1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
