Mitochondrial trafficking to synapses in cultured primary cortical neurons
- PMID: 16807333
- PMCID: PMC6673923
- DOI: 10.1523/JNEUROSCI.1012-06.2006
Mitochondrial trafficking to synapses in cultured primary cortical neurons
Abstract
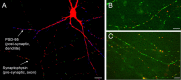
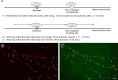
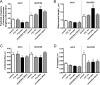
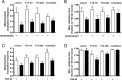
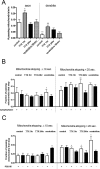
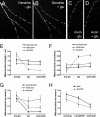
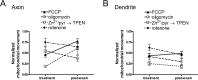
Functional synapses require mitochondria to supply ATP and regulate local [Ca2+]i for neurotransmission. Mitochondria are thought to be transported to specific cellular regions of increased need such as synapses. However, little is known about how this occurs, including the spatiotemporal distribution of mitochondria relative to presynaptic and postsynaptic sites, whether mitochondria are dynamically recruited to synapses, and how synaptic activity affects these trafficking patterns. We used primary cortical neurons in culture that form synaptic connections and show spontaneous synaptic activity under normal conditions. Neurons were cotransfected with a mitochondrially targeted cyan fluorescent protein and an enhanced yellow fluorescent protein-tagged synaptophysin or postsynaptic density-95 plasmid to label presynaptic or postsynaptic structures, respectively. Fluorescence microscopy revealed longer dendritic mitochondria that occupied a greater fraction of neuronal process length than axonal mitochondria. Mitochondria were significantly more likely to be localized at synaptic sites. Although this localization was unchanged by inhibition of synaptic activity by tetrodotoxin, it increased in dendritic synapses and decreased in axonal synapses during overactivity by veratridine. Mitochondrial movement and recruitment to synapses also differed between axons and dendrites under basal conditions and when synaptic activity was altered. Additionally, we show that movement of dendritic mitochondria can be selectively impaired by glutamate and zinc. We conclude that mitochondrial trafficking to synapses is dynamic in neurons and is modulated by changes in synaptic activity. Furthermore, mitochondrial morphology and distribution may be optimized differentially to best serve the synaptic distributions in axons and dendrites. Last, selective cessation of mitochondrial movement in dendrites suggests early postsynaptic dysfunction in neuronal injury and degeneration.
Figures
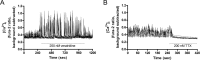
References
-
- Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA (2003). Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol 15:706–716. - PubMed
-
- Briones TL, Suh E, Jozsa L, Rogozinska M, Woods J, Wadowska M (2005). Changes in number of synapses and mitochondria in presynaptic terminals in the dentate gyrus following cerebral ischemia and rehabilitation training. Brain Res 1033:51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
