Hypoxic vasoconstriction of partial muscular intra-acinar pulmonary arteries in murine precision cut lung slices
- PMID: 16808843
- PMCID: PMC1524949
- DOI: 10.1186/1465-9921-7-93
Hypoxic vasoconstriction of partial muscular intra-acinar pulmonary arteries in murine precision cut lung slices
Abstract
Background: Acute alveolar hypoxia causes pulmonary vasoconstriction (HPV) which serves to match lung perfusion to ventilation. The underlying mechanisms are not fully resolved yet. The major vascular segment contributing to HPV, the intra-acinar artery, is mostly located in that part of the lung that cannot be selectively reached by the presently available techniques, e.g. hemodynamic studies of isolated perfused lungs, recordings from dissected proximal arterial segments or analysis of subpleural vessels. The aim of the present study was to establish a model which allows the investigation of HPV and its underlying mechanisms in small intra-acinar arteries.
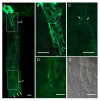
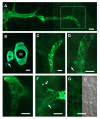
Methods: Intra-acinar arteries of the mouse lung were studied in 200 mum thick precision-cut lung slices (PCLS). The organisation of the muscle coat of these vessels was characterized by alpha-smooth muscle actin immunohistochemistry. Basic features of intra-acinar HPV were characterized, and then the impact of reactive oxygen species (ROS) scavengers, inhibitors of the respiratory chain and Krebs cycle metabolites was analysed.
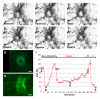
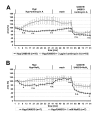
Results: Intra-acinar arteries are equipped with a discontinuous spiral of alpha-smooth muscle actin-immunoreactive cells. They exhibit a monophasic HPV (medium gassed with 1% O2) that started to fade after 40 min and was lost after 80 min. This HPV, but not vasoconstriction induced by the thromboxane analogue U46619, was effectively blocked by nitro blue tetrazolium and diphenyleniodonium, indicating the involvement of ROS and flavoproteins. Inhibition of mitochondrial complexes II (3-nitropropionic acid, thenoyltrifluoroacetone) and III (antimycin A) specifically interfered with HPV, whereas blockade of complex IV (sodium azide) unspecifically inhibited both HPV and U46619-induced constriction. Succinate blocked HPV whereas fumarate had minor effects on vasoconstriction.
Conclusion: This study establishes the first model for investigation of basic characteristics of HPV directly in intra-acinar murine pulmonary vessels. The data are consistent with a critical involvement of ROS, flavoproteins, and of mitochondrial complexes II and III in intra-acinar HPV. In view of the lack of specificity of any of the classical inhibitors used in such types of experiments, validation awaits the use of appropriate knockout strains and siRNA interference, for which the present model represents a well-suited approach.
Figures




Similar articles
-
Videomorphometric analysis of hypoxic pulmonary vasoconstriction of intra-pulmonary arteries using murine precision cut lung slices.J Vis Exp. 2014 Jan 14;(83):e50970. doi: 10.3791/50970. J Vis Exp. 2014. PMID: 24458260 Free PMC article.
-
Mitochondrial complex II is essential for hypoxia-induced pulmonary vasoconstriction of intra- but not of pre-acinar arteries.Cardiovasc Res. 2012 Mar 15;93(4):702-10. doi: 10.1093/cvr/cvr359. Epub 2012 Jan 2. Cardiovasc Res. 2012. PMID: 22215723
-
Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing.Circ Res. 2001 Jun 22;88(12):1259-66. doi: 10.1161/hh1201.091960. Circ Res. 2001. PMID: 11420302
-
Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells.J Mol Cell Cardiol. 2004 Dec;37(6):1119-36. doi: 10.1016/j.yjmcc.2004.09.007. J Mol Cell Cardiol. 2004. PMID: 15572043 Review.
-
Hypoxic pulmonary vasoconstriction: mechanisms and controversies.J Physiol. 2006 Jan 1;570(Pt 1):53-8. doi: 10.1113/jphysiol.2005.098855. Epub 2005 Oct 27. J Physiol. 2006. PMID: 16254010 Free PMC article. Review.
Cited by
-
Integrative understanding of hypoxic pulmonary vasoconstriction using in vitro models: from ventilated/perfused lung to single arterial myocyte.Integr Med Res. 2014 Dec;3(4):180-188. doi: 10.1016/j.imr.2014.08.003. Epub 2014 Sep 3. Integr Med Res. 2014. PMID: 28664095 Free PMC article. Review.
-
Intermedin stabilized endothelial barrier function and attenuated ventilator-induced lung injury in mice.PLoS One. 2012;7(5):e35832. doi: 10.1371/journal.pone.0035832. Epub 2012 May 1. PLoS One. 2012. PMID: 22563471 Free PMC article.
-
Hypoxia-induced endothelial CX3CL1 triggers lung smooth muscle cell phenotypic switching and proliferative expansion.Am J Physiol Lung Cell Mol Physiol. 2012 Nov 15;303(10):L912-22. doi: 10.1152/ajplung.00014.2012. Epub 2012 Sep 21. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 23002075 Free PMC article.
-
Pre-clinical assessment of a water-in-fluorocarbon emulsion for the treatment of pulmonary vascular diseases.Drug Deliv. 2019 Dec;26(1):147-157. doi: 10.1080/10717544.2019.1568621. Drug Deliv. 2019. PMID: 30822171 Free PMC article.
-
Vascular Dysfunction in Pneumocystis-Associated Pulmonary Hypertension Is Related to Endothelin Response and Adrenomedullin Concentration.Am J Pathol. 2016 Feb;186(2):259-69. doi: 10.1016/j.ajpath.2015.10.008. Epub 2015 Dec 11. Am J Pathol. 2016. PMID: 26687815 Free PMC article.
References
-
- von Euler U, Liljestrand G. Observations on pulmonary arterial blood pressure in cat. Acta Physiol Scand. 1946;12:301–320.
-
- Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. - PubMed
-
- Yamagucchi K, Suzuki K, Naoki K, Nishio K, Sato N, Takewshita K, Kudo H, Aoki T, Suzuki Y, Miyata A, Tsumura H. Response of intra-acinar pulmonary microvessels to hypoxia, hypercapnic acidosis, and isocapnic acidosis. Circ Res. 1998;82:722–728. - PubMed
-
- Grimminger F, Spriestersbach R, Weissmann N, Walmrath D, Seeger W. Nitric oxide generation and hypoxic vasoconstriction in buffer-perfused rabbit lungs. J Appl Physiol. 1995;78:1509–1515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

