A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts
- PMID: 16808845
- PMCID: PMC1524957
- DOI: 10.1186/1746-4811-2-13
A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts
Abstract
Background: Transient assays using protoplasts are ideal for processing large quantities of genetic data coming out of hi-throughput assays. Previously, protoplasts have routinely been prepared from dicot tissue or cell suspension cultures and yet a good system for rice protoplast isolation and manipulation is lacking.
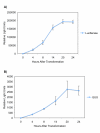
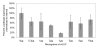
Results: We have established a rice seedling protoplast system designed for the rapid characterization of large numbers of genes. We report optimized methods for protoplast isolation from 7-14 day old etiolated rice seedlings. We show that the reporter genes luciferase GL2 and GUS are maximally expressed approximately 20 h after polyethylene glycol (PEG)-mediated transformation into protoplasts. In addition we found that transformation efficiency varied significantly with plasmid size. Five micrograms of a 4.5 kb plasmid resulted in 60-70% transformation efficiency. In contrast, using 50 microg of a 12 kb plasmid we obtained a maximum of 25-30% efficiency. We also show that short interfering RNAs (siRNAs) can be used to silence exogenous genes quickly and efficiently. An siRNA targeting luciferase resulted in a significant level of silencing after only 3 hours and up to an 83% decrease in expression. We have also isolated protoplasts from cells prepared from fully green tissue. These green tissue-derived protoplasts can be transformed to express high levels of luciferase activity and should be useful for assaying light sensitive cellular processes.
Conclusion: We report a system for isolation, transformation and gene silencing of etiolated rice leaf and stem-derived protoplasts. Additionally, we have extended the technology to protoplasts isolated from fully green tissue. The protoplast system will bridge the gap between hi-throughput assays and functional biology as it can be used to quickly study large number of genes for which the function is unknown.
Figures




References
-
- De Sutter V, Vanderhaeghen R, Tilleman S, Lammertyn F, Vanhoutte I, Karimi M, Inze D, Goossens A, Hilson P. Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J. 2005;44:1065–1076. doi: 10.1111/j.1365-313X.2005.02586.x. - DOI - PubMed
-
- Cocking EC. The isolation of plant protoplasts. Methods Enzymol. 1974;31:578–583. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

