Epstein-Barr virus LMP2A enhances B-cell responses in vivo and in vitro
- PMID: 16809282
- PMCID: PMC1489056
- DOI: 10.1128/JVI.00433-06
Epstein-Barr virus LMP2A enhances B-cell responses in vivo and in vitro
Abstract
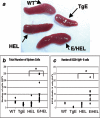
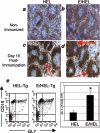
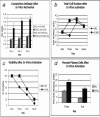
Epstein-Barr virus (EBV) establishes latent infections in a significant percentage of the population. Latent membrane protein 2A (LMP2A) is an EBV protein expressed during latency that inhibits B-cell receptor signaling in lymphoblastoid cell lines. In the present study, we have utilized a transgenic mouse system in which LMP2A is expressed in B cells that are specific for hen egg lysozyme (E/HEL-Tg). To determine if LMP2A allows B cells to respond to antigen, E/HEL-Tg mice were immunized with hen egg lysozyme. E/HEL-Tg mice produced antibody in response to antigen, indicating that LMP2A allows B cells to respond to antigen. In addition, E/HEL-Tg mice produced more antibody and an increased percentage of plasma cells after immunization compared to HEL-Tg littermates, suggesting that LMP2A increased the antibody response in vivo. Finally, in vitro studies determined that LMP2A acts directly on the B cell to increase antibody production by augmenting the expansion and survival of the activated B cells, as well as increasing the percentage of plasma cells generated. Taken together, these data suggest that LMP2A enhances, not diminishes, B-cell-specific antibody responses in vivo and in vitro in the E/HEL-Tg system.
Figures

Similar articles
-
Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen.J Virol. 2005 Jun;79(12):7355-62. doi: 10.1128/JVI.79.12.7355-7362.2005. J Virol. 2005. PMID: 15919890 Free PMC article.
-
Epstein-Barr virus LMP2A imposes sensitivity to apoptosis.J Gen Virol. 2010 Sep;91(Pt 9):2197-202. doi: 10.1099/vir.0.021444-0. Epub 2010 May 19. J Gen Virol. 2010. PMID: 20484564 Free PMC article.
-
The effects of the Epstein-Barr virus latent membrane protein 2A on B cell function.Int Rev Immunol. 2001;20(6):805-35. doi: 10.3109/08830180109045591. Int Rev Immunol. 2001. PMID: 11913951 Review.
-
Hen egg-white lysozyme-specific T cells elicited in hen egg-white lysozyme-transgenic mice retain an imprint of self-tolerance.J Immunol. 1993 Sep 15;151(6):3057-69. J Immunol. 1993. PMID: 8376768
-
Epstein-Barr virus: exploiting the immune system.Nat Rev Immunol. 2001 Oct;1(1):75-82. doi: 10.1038/35095584. Nat Rev Immunol. 2001. PMID: 11905817 Review.
Cited by
-
Functional interplay of Epstein-Barr virus oncoproteins in a mouse model of B cell lymphomagenesis.Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14421-14432. doi: 10.1073/pnas.1921139117. Epub 2020 Jun 10. Proc Natl Acad Sci U S A. 2020. PMID: 32522871 Free PMC article.
-
Transcriptome changes induced by Epstein-Barr virus LMP1 and LMP2A in transgenic lymphocytes and lymphoma.mBio. 2012 Sep 18;3(5):e00288-12. doi: 10.1128/mBio.00288-12. Print 2012. mBio. 2012. PMID: 22991431 Free PMC article.
-
The impact of Epstein-Barr virus latent membrane protein 2A on the production of B cell activating factor of the tumor necrosis factor family (BAFF), APRIL and their receptors.Immun Inflamm Dis. 2022 Nov;10(11):e729. doi: 10.1002/iid3.729. Immun Inflamm Dis. 2022. PMID: 36301035 Free PMC article.
-
Mouse model of Epstein-Barr virus LMP1- and LMP2A-driven germinal center B-cell lymphoproliferative disease.Proc Natl Acad Sci U S A. 2017 May 2;114(18):4751-4756. doi: 10.1073/pnas.1701836114. Epub 2017 Mar 28. Proc Natl Acad Sci U S A. 2017. PMID: 28351978 Free PMC article.
-
Epstein-Barr virus LMP2A increases IL-10 production in mitogen-stimulated primary B-cells and B-cell lymphomas.J Gen Virol. 2013 May;94(Pt 5):1127-1133. doi: 10.1099/vir.0.049221-0. Epub 2013 Jan 9. J Gen Virol. 2013. PMID: 23303827 Free PMC article.
References
-
- Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. - PubMed
-
- Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405-411. - PubMed
-
- Casola, S., K. L. Otipoby, M. Alimzhanov, S. Humme, N. Uyttersprot, J. L. Kutok, M. C. Carroll, and K. Rajewsky. 2004. B cell receptor signal strength determines B cell fate. Nat. Immunol. 5:317-327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

