Evaluating replication-defective vesicular stomatitis virus as a vaccine vehicle
- PMID: 16809305
- PMCID: PMC1489030
- DOI: 10.1128/JVI.00365-06
Evaluating replication-defective vesicular stomatitis virus as a vaccine vehicle
Abstract
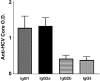
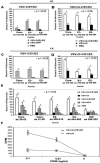
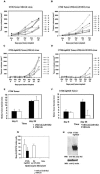
We have generated replication-competent (VSV-C/E1/E2) and nonpropagating (VSVDeltaG-C/E1/E2) vesicular stomatitis virus (VSV) contiguously expressing the structural proteins of hepatitis C virus (HCV; core [C] and glycoproteins E1 and E2) and report on their immunogenicity in murine models. VSV-C/E1/E2 and VSVDeltaG-C/E1/E2 expressed high levels of HCV C, E1, and E2, which were authentically posttranslationally processed. Both VSV-expressed HCV E1-E2 glycoproteins were found to form noncovalently linked heterodimers and appeared to be correctly folded, as confirmed by coimmunoprecipitation analysis using conformationally sensitive anti-HCV-E2 monoclonal antibodies (MAbs). Intravenous or intraperitoneal immunization of BALB/c mice with VSV-C/E1/E2 or VSVDeltaG-C/E1/E2 resulted in significant and surprisingly comparable HCV core or E2 antibody responses compared to those of control mice. In addition, both virus types generated HCV C-, E1-, or E2-specific gamma interferon (IFN-gamma)-producing CD8(+) T cells, as determined by enzyme-linked immunospot (ELISPOT) analysis. Mice immunized with VSVDeltaG-C/E1/E2 were also protected against the formation of tumors expressing HCV E2 (CT26-hghE2t) and exhibited CT26-hghE2t-specific IFN-gamma-producing and E2-specific CD8(+) T-cell activity. Finally, recombinant vaccinia virus (vvHCV.S) expressing the HCV structural proteins replicated at significantly lower levels when inoculated into mice immunized with VSV-C/E1/E2 or VSVDeltaG-C/E1/E2, but not with control viruses. Our data therefore illustrate that potentially safer replication-defective VSV can be successfully engineered to express high levels of antigenically authentic HCV glycoproteins. In addition, this strategy may therefore serve in effective vaccine and immunotherapy-based approaches to the treatment of HCV-related disease.
Figures





Similar articles
-
Virus-Like Vesicle-Based Therapeutic Vaccine Vectors for Chronic Hepatitis B Virus Infection.J Virol. 2015 Oct;89(20):10407-15. doi: 10.1128/JVI.01184-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246574 Free PMC article.
-
Efficient formation of vesicular stomatitis virus pseudotypes bearing the native forms of hepatitis C virus envelope proteins detected after sonication.Microbes Infect. 2005 Jan;7(1):29-40. doi: 10.1016/j.micinf.2004.09.006. Epub 2004 Dec 9. Microbes Infect. 2005. PMID: 15716060
-
Generation of hepatitis C virus-like particles by use of a recombinant vesicular stomatitis virus vector.J Virol. 2002 Dec;76(23):12325-34. doi: 10.1128/jvi.76.23.12325-12334.2002. J Virol. 2002. PMID: 12414973 Free PMC article.
-
[Vesicular stomatitis virus (VSV) as a vaccine vector for immunization against viral infections].Postepy Hig Med Dosw (Online). 2013 Jan 11;67:1345-58. doi: 10.5604/17322693.1083016. Postepy Hig Med Dosw (Online). 2013. PMID: 24379275 Review. Polish.
-
Hepatitis C virus envelope glycoproteins and potential for vaccine development.Vox Sang. 2002 Aug;83 Suppl 1:27-32. doi: 10.1111/j.1423-0410.2002.tb05262.x. Vox Sang. 2002. PMID: 12617098 Review.
Cited by
-
Plant Viruses in Plant Molecular Pharming: Toward the Use of Enveloped Viruses.Front Plant Sci. 2019 Jun 19;10:803. doi: 10.3389/fpls.2019.00803. eCollection 2019. Front Plant Sci. 2019. PMID: 31275344 Free PMC article. Review.
-
Heat shock protein 70 enhances mucosal immunity against human norovirus when coexpressed from a vesicular stomatitis virus vector.J Virol. 2014 May;88(9):5122-37. doi: 10.1128/JVI.00019-14. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574391 Free PMC article.
-
Replicating and non-replicating viral vectors for vaccine development.Curr Opin Biotechnol. 2007 Dec;18(6):546-56. doi: 10.1016/j.copbio.2007.10.010. Epub 2007 Dec 11. Curr Opin Biotechnol. 2007. PMID: 18063357 Free PMC article. Review.
-
Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses.Gene Ther. 2013 Mar;20(3):255-61. doi: 10.1038/gt.2012.31. Epub 2012 Apr 5. Gene Ther. 2013. PMID: 22476202 Free PMC article.
-
A recombinant DNA and vaccinia virus prime-boost regimen induces potent long-term T-cell responses to HCV in BALB/c mice.Vaccine. 2009 Mar 26;27(15):2085-8. doi: 10.1016/j.vaccine.2009.02.003. Epub 2009 Feb 12. Vaccine. 2009. PMID: 19356609 Free PMC article.
References
-
- Adams, D. O., T. Hall, Z. Steplewski, and H. Koprowski. 1984. Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype contain increased numbers of macrophages activated for a distinctive form of antibody-dependent cytolysis. Proc. Natl. Acad. Sci. USA 81:3506-3510. - PMC - PubMed
-
- Afdhal, N. H. 2004. The natural history of hepatitis C. Semin. Liver Dis. 24(Suppl. 2):3-8. - PubMed
-
- Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135-138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

