Deregulation of eIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein
- PMID: 16809313
- PMCID: PMC1489051
- DOI: 10.1128/JVI.02380-05
Deregulation of eIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein
Abstract
Infections with high-risk human papillomaviruses (HPVs) are linked to more than 95% of cervical cancers. HPVs replicate exclusively in differentiated cells and the function of the HPV E7 oncoprotein is essential for viral replication. In this study, we investigated the mechanism that regulates E7 expression in differentiated cells. The level of E7 protein was strongly induced in HPV-containing Caski, HOK-16B, and BaP-T cells during growth in methylcellulose-containing medium, a condition that induces differentiation. Enhanced expression of E7 was observed between 4 and 8 h of culturing in methylcellulose and was maintained for up to 24 h. The increase was not due to altered stability of the E7 protein or an increase in the steady-state level of the E7 mRNA. Instead, the translation of the E7 mRNA was enhanced during differentiation. More than 70 to 80% of the E7 mRNA was found in the polysome fractions in the differentiated cells. Consistent with this observation, higher levels of the phosphorylated translator inhibitor 4E-BP1 were observed in differentiated HPV-containing cells but not in differentiated non-HPV tumor cells or primary keratinocytes. The mTOR kinase inhibitor rapamycin blocked phosphorylation of 4E-BP1 and significantly decreased the level of E7 protein in Caski cells, suggesting that phosphorylation of 4E-BP1 is linked to E7 expression. Prevailing models for the molecular mechanisms underlying E7 expression have focused largely on transcriptional regulation. The results presented in this study demonstrate a significant role of the cellular translation machinery to maintain a high level of E7 protein in differentiated cells.
Figures


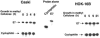




References
-
- Avdulov, S., S. Li, V. Michalek, D. Burrichter, M. Peterson, D. M. Perlman, J. C. Manivel, N. Sonenberg, D. Yee, P. B. Bitterman, and V. A. Polunovsky. 2004. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell 5:553-563. - PubMed
-
- Berezutskaya, E., A. Morozov, P. Raychaudhuri, and S. Bagchi. 1997. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 8:1277-1286. - PubMed
-
- Bjornsti, M. A., and P. J. Houghton. 2004. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell 5:519-523. - PubMed
-
- Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papillomavirus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

