Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling
- PMID: 16809315
- PMCID: PMC1489049
- DOI: 10.1128/JVI.02579-05
Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling
Abstract
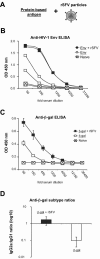
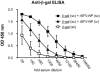
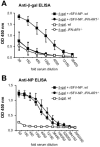
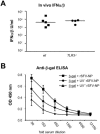
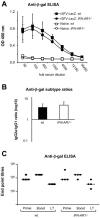
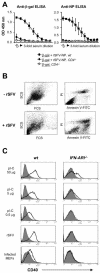
Viruses typically elicit potent adaptive immune responses, and live-virus-based vaccines are among the most efficient human vaccines known. The mechanisms by which viruses stimulate adaptive immune responses are not fully understood, but activation of innate immune signaling pathways in the early phase of the infection may be of importance. In addition to stimulating immune responses to viral antigens expressed in infected cells, viruses can also provide adjuvant signals to coimmunized protein antigens. Using recombinant Semliki Forest virus (rSFV)-based vaccines, we show that rSFV potently enhanced antibody responses against coimmunized protein antigens in the absence of other exogenously added adjuvants. Elicitation of antibody responses against both virus-encoded antigens and coimmunized protein antigens was independent of the signaling via Toll-like receptors (TLRs) previously implicated in antiviral responses. In contrast, the adjuvant effect of rSFV on coimmunized protein was completely abolished in mice lacking the alpha/beta interferon (IFN-alpha/beta) receptor (IFN-AR1), demonstrating that IFN-alpha/beta signaling was critical for mediating this effect. Antibody responses directed against virus-encoded antigens were intact in IFN-AR1(-/-) mice, suggesting that other signals are sufficient to drive immune responses against virally encoded antigens. These data provide a basis for the adjuvant effect of rSFV and show that different signals are required to stimulate antibody responses to virally encoded antigens and to antigens administered as purified protein vaccines, together with viral particles.
Figures
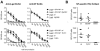
References
-
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Alsharifi, M., M. Lobigs, M. Regner, E. Lee, A. Koskinen, and A. Mullbacher. 2005. Type I interferons trigger systemic, partial lymphocyte activation in response to viral infection. J. Immunol. 175:4635-4640. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljeström. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17:497-507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

