Transmission of simian immunodeficiency virus SIVcpz and the evolution of infection in the presence and absence of concurrent human immunodeficiency virus type 1 infection in chimpanzees
- PMID: 16809326
- PMCID: PMC1489021
- DOI: 10.1128/JVI.00382-06
Transmission of simian immunodeficiency virus SIVcpz and the evolution of infection in the presence and absence of concurrent human immunodeficiency virus type 1 infection in chimpanzees
Abstract
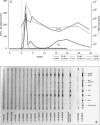
Current data suggest that the human immunodeficiency virus type 1 (HIV-1) epidemic arose by transmission of simian immunodeficiency virus (SIV) SIVcpz from a subspecies of common chimpanzees (Pan troglodytes troglodytes) to humans. SIVcpz of chimpanzees is itself a molecular chimera of SIVs from two or more different monkey species, suggesting that recombination was made possible by coinfection of one individual animal with different lentiviruses. However, very little is known about SIVcpz transmission and the susceptibility to lentivirus coinfection of its natural host, the chimpanzee. Here, it is revealed that either infected plasma or peripheral blood mononuclear cells readily confer infection when exposure occurs by the intravenous or mucosal route. Importantly, the presence of preexisting HIV-1 infection did not modify the kinetics of SIVcpz infection once it was established by different routes. Although humoral responses appeared as early as 4 weeks postinfection, neutralization to SIVcpz-ANT varied markedly between animals. Analysis of the SIVcpz env sequence over time revealed the emergence of genetic viral variants and persistent SIVcpz RNA levels of between 10(4) and 10(5) copies/ml plasma regardless of the presence or absence of concurrent HIV-1 infection. These unique data provide important insight into possible routes of transmission, the kinetics of acute SIVcpz infection, and how readily coinfection with SIVcpz and other lentiviruses may be established as necessary preconditions for potential recombination.
Figures




References
-
- Bailes, E., F. Gao, F. Bibollet-Ruche, V. Courgnaud, M. Peeters, P. A. Marx, B. H. Hahn, and P. M. Sharp. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. - PubMed
-
- Balla-Jhagjhoorsingh, S., P. Mooij, G. Koopman, T. Haaksma, V. Teeuwsen, J. Heeney, and R. Bontrop. 1999. Differential cytotoxic T-lymphocyte (CTL) responses in HIV-1 immunised sibling chimpanzees with shared MHC haplotypes. Immunol. Lett. 66:61-67. - PubMed
-
- Balla-Jhagjhoorsingh, S. S., G. Koopman, P. Mooij, T. G. Haaksma, V. J. Teeuwsen, R. E. Bontrop, and J. L. Heeney. 1999. Conserved CTL epitopes shared between HIV-infected human long-term survivors and chimpanzees. J. Immunol. 162:2308-2314. - PubMed
-
- Beirnaert, E., B. Willems, M. Peeters, A. Bouckaert, L. Heyndrickx, P. Zhong, K. Vereecken, S. Coppens, D. Davis, P. Ndumbe, W. Janssens, and G. van der Groen. 1998. Design and evaluation of an in-house HIV-1 (group M and O), SIVmnd and SIVcpz antigen capture assay. J. Virol. Methods 73:65-70. - PubMed
-
- Bogers, W. M., W. H. Koornstra, R. H. Dubbes, P. J. ten Haaft, B. E. Verstrepen, S. S. Jhagjhoorsingh, A. G. Haaksma, H. Niphuis, J. D. Laman, S. Norley, H. Schuitemaker, J. Goudsmit, G. Hunsmann, J. L. Heeney, and H. Wigzell. 1998. Characteristics of primary infection of a European human immunodeficiency virus type 1 clade B isolate in chimpanzees. J. Gen. Virol. 79:2895-2903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

